-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Sociological Research
p-ISSN: 2166-5443 e-ISSN: 2166-5451
2013; 3(2): 7-16
doi:10.5923/j.sociology.20130302.01
Ethics of Empirical Qualitative Research in Construction Health and Safety: Reflections From a PhD Project Part 1
Manikam Pillay
School of Health Sciences, University of Ballarat, Victoria, 3353, Australia
Correspondence to: Manikam Pillay, School of Health Sciences, University of Ballarat, Victoria, 3353, Australia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The increased use of empirical qualitative health and safety research involving humans in contemporary industrial domains such as construction means a high degree of attention is being paid to research process; however less attention is paid to the ethical issues of the conduct of such research. In the main, it is generally understood that all qualitative researchers will follow established rules and guidelines as laid down by institute research boards and research agencies, through an approval process prior to the conduct of research. However, ethical issues permeate the entire qualitative research process; yet there is little in terms of published literatures that discuss how this important issue was actually addressed by the researchers as part of the research process. This is a significant gap in the literature in empirical qualitative health and safety research. This paper, one of two articles, is an attempt to narrow this gap. It reflects on ethical issues that were considered during planning, access and entry, and how these and other issues not considered and planned for addressed as part of the research project.
Keywords: Empirical Qualitative Research, Construction Health and Safety, PLIS
Cite this paper: Manikam Pillay, Ethics of Empirical Qualitative Research in Construction Health and Safety: Reflections From a PhD Project Part 1, American Journal of Sociological Research, Vol. 3 No. 2, 2013, pp. 7-16. doi: 10.5923/j.sociology.20130302.01.
Article Outline
1. Introduction
- Health and safety is an important area of research and practice in both developed and developing countries, and recent years have seen an increased number of papers being published in this field. Special issues of such research in construction[1]and the continued publication of such papers across this industrial domain in many journals and conferences proceedings serve to reinforce this point. Many of published papers suggest qualitative research and humans were the main sources of data on a range of topics, for example, on safety culture[2-5], design and implementation[6, 7], barriers for technical controls[8], and workers behaviour[9]. The increased use of qualitative research involving humans means there is a high degree of attention being paid to research process, but less attention is given to the ethical issues associated with the conduct of such research[10-12]. Most published theses merely mention the fact that ethics approval was received from the appropriate institutional human ethics research committee (IHREC), creating an assumption that to do qualitative research successfully, it is sufficient to follow the rules and guidelines stipulated by IHRECs and gain their approval for conducting the research. However, ethical issues permeate the entire research process; yet very little is known about the actual practice of ethics in research[11]; in particular those which were qualitative in nature[12, 13].This leaves novice researchers and research students ill-prepared to deal with ethical issues across their research journey. Therest of paper is organised as follows. First, existing research on the issues surrounding ethical research on human is discussed. Next, qualitative research is introduced, followed by a background to this research. It then discusses the ethical issues that were considered at two stages of the research process, planning and recruitment.
2. Literature Review
- A review of the above literature suggests that ethics issues have been published from a range of domains such as nursing[12, 14, 15], palliative care[16], healthcare[13, 17] and medicine[18, 19]. Works have also been published on the development of ethics research policies[20-23] and ethics committees[24, 25]. Procedures and guidelines used in a number of countries such as Australia[22, 23, 26], Sweden[27] and Sudan[28] have also been discussed. Research has also been published on specific ethical issues such as informed consent[29-32], incentives[33] and privacy and confidentiality[34]. Integrative reviews on ethics have looked at methodological and ethics associated with research on children and young people[15], professional and occupational responsibilities[35], linking ethics, research practice and epistemology[36] and the links between quality and ethics[37]. Kirby[38] argues that the key principles governing informed consent required that, if the procedure being used had a reasonable possibility of harm to the patient, these were fully explained to him/her prior to any diagnostic or therapeutic procedure being carried out. He also argued that any informed consent obtained after the procedure had been used required the patient to be adequately instructed about the ratio of risk and benefit with the procedure, in comparison to any alternative procedures or no treatment at all.de Raeve[16] reviewed ethical issues associated with using participant observation, experiments and unstructured interviewed methods for collecting data from vulnerable participants who were in palliative care (cancer patients who were no longer capable of responding to treatments and most likely to die within six months). According to the researcher, experimental methods treated the participant as a statistic only, was ruthless and impersonal, so the consideration of harm to participant was a non-issue; however, this could create potential role-conflicts as a carer and researcher. The author also argued that participant observations and semi-structured created an illusion of collaboration whilecreating an uneven distribution of power in reality; moreover, moral values and judgement on the part of the researcher were important about how research in such settings were carried out.O'Neill[32] discusses some of the limits of informed consent as used in medical research, arguing that while this is designed to support autonomy, there are many distinct conceptions of individual autonomy, hence their importance varied. He argued that a better reason for taking informed consent seriously was that it provided an assurance against deception and coercion. Furthermore, because consent was a propositional attitude, the achievement of wholly specific consent was an illusion, the approach utilised should be designed to give patients and others control over the amount of information they received, and the opportunity to rescind consent they had already given.Grant and Sugarman[33] examined the advantages and disadvantages of using incentives to induce participation in research. Writing about their experiences in medical research, the authors argued that when the research the met the usual criteria for human subject research, the providing of incentives themselves was a benign issue. However, they would become problematic where the research participant(s) were in a dependent relationship with the researcher, where the risks were relatively high, where the research would be deemed degrading, where participants were only willing to consent if the incentives were relatively large because of their strong aversion to the study. The authors offer a set of guidelines for judging the ethical merits of incentives in research.Kottow[39] argued that the value of informed consent in research on human subjects and informed decision in clinical settings was constantly being eroded as a result of coming under constant fire, most of which was heavily influenced by ad hoc considerations that sought to reduce patient/subject autonomy in order to expedite biomedical investigations and clinical decisions easier. He suggested that a pathic/proleptic standard was required, whereby the patient or research subject received all the information that may be required at the time most likely to them. Under this standardit was the participant who decided how they were affected, but it remained the duty of the researcher to inform them of how the materials gathered would be used in future.Kapp[40] examined the legal and ethical issues associated with the handling of human tissues for pathological research. His argument centred round the issues of informed consent (particularly for storage of organs) and confidentiality, and suggested that pathologists needed to be cognisant of both existing regulations and policies that were relevant in their states for storing tissues that were likely to be used studies in the future. Mitchell and Irvine[41] reflected on a UK government funded study that explored mental health and employment. Some of the key issues they dealt with included consent and control, rapport building, managing and responding to emotion, and offering appropriate longer term support. The researchers discussed their personal approaches and experiences (practical, methodological, ethical) during and after the fieldwork process, highlighting some of the challenges they faced and discuss how these were addressed and managed. They make the point that researchers needed to be aware of their “research footprint,” in particular the need to be reflexive and responsive to participants’ emotional wellbeing.Murphy and Dingwall[42] examine informed consent as applied to ethnographic research in health care settings. In their article they challenge the notion of informed consent as practiced in bureaucratic practices of ethical reviews, and argue that these anticipatory regulatory regimes threatened the significant contribution of ethnographic research to the creation of more efficient, more effective, more equitable and more humane health care systems. Furthermore, they suggest that informed consent in ethnographic research was neither achievable nor demonstrable in the terms set by anticipatory regulatory regimes that take clinical research or biomedical experimentation as their paradigm cases, and suggest there was a need to develop and strengthen professional models of regulation based on education, training and mutual accountabilityThe above series of published research are based on medical and healthcare settings. As argued by Richards and Schwartz[13], while there was significant guidance on ethics involving medical research these were largely aimed at quantitative researchers, with little attention paid to qualitative research. The researchers suggested qualitative health researchers needed to be aware of four key ethical issues, including (i) anxiety and distress, (ii) exploitation, (iii) misrepresentation and (iv) identification of participants in published papers[13]. They recommended risks of harm to participants could be reduced through scientific soundness (proper design, adequate expertise and supervision); organising follow-up care where appropriate, a consideration of seeking informed consent, ensuring confidentiality and reflexivity during data analysis. The authors argued that while their recommendations sounded over-prescriptive, inadequate or impractical, they were necessary for health services research because the actual risks to participants were unknown.Robley[14] suggested that decisions about what to study, the selection of participants, the methodology that was employed, means of truly achieving informed consent, decisions about when to terminate or interrupt interviews or to probe more deeply, when nursing care preceded the research, and what and how case studies were to be documented were some of the key ethical concerns qualitative nursing researchers needed to be aware of. Orb, et al.[12]argued that ethical issues relevant in health research included appropriateness of research design, methodological design, funding sources and behaviours in the reporting of data. They posit that ethical difficulties could be reduced through an awareness and application of ethical principles such as autonomy, beneficence and justice[12]. The authors suggest a number of strategies such as informed consent (or negotiation of trust), using pseudonyms, restricting circulation of report or paper, seeking consent and/or making aware to participants that concepts of ideas they have used may be used in published works, and giving a voice to minorities, disadvantaged groups and protecting vulnerable participants such as children, prisoners, mentally ill and elderly[12]. The authors acknowledge that the principles they had advocated may not necessarily ensure ethical research but contribute to an understanding that ethical responsibility in qualitative research was an ongoing process. Soobrayan[43] reviewed ethics, trust and politics associated with constructivist qualitative research, and argued that these were essentially a confrontation one’s self as a researcher which needed to be recognised. Moreover, she argued that there was no single set of rules or practices that could be used to govern such research because these were contextually driven, requiring the qualitative researcher to constantly and consistently to deliberate and engage with throughout the research process.In a Swedish longitudinal study, Helgesson, et al.[29]identified that that many of the research subjects had a limited understanding of the study, despite this they were still satisfied with their understanding and sufficient enough for informed continued participation.The authors arguedthat the kind of understanding morally required depended partly on the kind of understanding onwhich the research subjects wanted to base their decisions, and partly on what kind of knowledge they lacked. They suggested that (i) researchers needed ensure that the information process was not flawed,(ii) participants received theinformation they wanted, (iii) new information efforts may be required, and (iv)researchershad to ensure participants had knowledge about those aspects that were important to them. They also found that the lack of knowledge among participants could also be attributed to conscious choices by research participants to disregard information deemed not important to them, and that these choices should be respected[29].Khanlou and Peter[44] discuss the ethical issues associated with conducting participatory action research, arguing that there were several overlaps between guidelines established for clinical and health promotion research. They posit however, that the use ofan itemized and list-oriented approach to evaluating the ethical merits of a PAR proposal was ineffective; it was more useful to evaluate the ethics proposals by relating them to specific criteria used for the evaluation of other health studies.Wiles, et al.[45] reviewed ethical issues associated withinformed consent in social research with one’s peers. The researchers identified the key issues were related to consent, data ownership and the management of confidentiality and anonymity, arguing that the increased regulation of research meant thatresearchers were required to attend reflexively to the social context in which consent was likely to occur.Morse[46] provides a set of guidelines she suggests would be useful in conducting qualitative health research in an ethical manner, including (i) having a priority for medical or emergency care over research activities, (ii) acting when patient safety is jeopardised, (iii) conflicting roles of researcher and practitioner as an observer and participants in one’s own work environment or settings, (iv) conflicts between research and therapeutic goals, (v) data collection which could violate anonymity, confidentiality and privacy, and (vi) researcher’s safety. These findings suggest that (i) qualitative research outside of health and medical settings may require researchers to be mindful and cognisant of a different set of ethical obligations; (ii) these obligations may transgress both legal and moral values and principles, (iii) researchers may be faced with a range of different ethical issues across the research journey, from conception to completion, and (iii) guidelines and principles, whilst being useful, were not necessary a panacea for ethical conduct of research, andWhat is missing in the literature are the actual experiences of researchers as they engage with the ethics process across their research journey.What appears to be missing in the literature is (1) research on ethics from tradition contexts such as agriculture, construction, manufacturing and mining, (2) ethicsinvolving health and safety research, and (3) actual experiences associated with the practice of qualitative research ethics. The aim of this paper is to make a start towards addressing some of these gaps.The next section gives an overview of qualitative research.
3. Introducing Qualitative Research
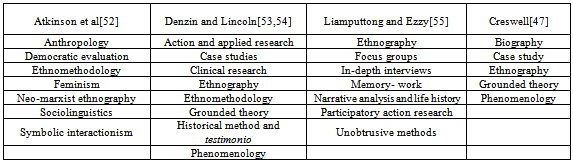
- Qualitative research is a form of investigation that (i) seeks answers to a question, (ii) systematically uses a predefined set of procedures to answer research question(s), (iii) collects evidence, (iv); produces findings thatare not determined in advance, and (v) are applicable beyond the immediate boundaries of the study[47, 48]. Moreover, it seeks to understand phenomena from the perspectives of people in naturalistic settings[49], involves recruiting people, obtaining getting data through interviews, questionnaires and observations, and reporting these in a manner that best seeks to represent the voice of the participants[50, 51]. There are many genres of qualitative research, with different authors describing them in different ways. For example Atkinson et al.[52] suggest there are at least seven (anthropology, democratic evaluation, ethnomethodoloy, feminism, neo-marxist ethnography, sociolinguists, and symbolic interactionism); whileDenzin and Lincoln[53, 54] expands these into eight (action and applied research, case studies, clinical research, ethnography, grounded theory, historical method, life history and testimonio, phenomenology ethnomethodology). Others such as Liamputtong and Ezzy[55]also suggested seven (ethnography, focus groups, in-depth interviews, memory-work, narrative analysis and life history, participatory action research and unobtrusive methods). Most recently Creswell[47, 50]has consolidated these into five (biography, case study, ethnography, grounded theory and phenomenology). Table 1 summarises these various genres.In this research the five system classification suggested by Creswell[47] was deemed useful, and the specific method used included case study.
4. Background to Research
- The research on which this article is based upon sought to explore safe work method statements (SWMS) in the Victorian construction industry within the framework of resilience engineering, a recent innovation in health and safety[56]. The purpose of this research was to develop an understanding of whether SWMS enhanced or hindered RE as health and safety management strategy, andthe research design chosen involved multi-level analysis of socio-technical organizational systems[57, 58]and qualitative case studies[47]with primary data collected through a triangulation[59]of semi-structured interviews, document analysis and field observation. Similar to most qualitative researchers, approval to conduct the study was sought and received from the University’s Human Research Ethics Committee (UHREC) prior to the collection and analysis of data. This required the researcher to identify hazards and assess risks of the project, and submit a written proposal that covered:1. An outline of key aspects,2. Aims and significance,3. Funding and financial benefits,4. Approvals5. Methods of data collection, requirements of participants, time commitments and analysis of data,6. Procedures for recruiting participants,7. Risk management procedures,8. Incentives for participation,9. Protection of information, and10. Legal issuesThe protocols are similar to those followed by most Western universities and research institutions[60].
5. Ethical Issues Considered During Planning
- The approval was granted based on ethical issues considered during the planning of the study; this included protection of participants from harm, seeking informed consent, privacy and confidentiality of data collected. The principal guiding document that was used to inform this was the National Statement on Ethical Conduct in Human Research[23].
5.1. Ethics of Research Design
- The first ethical that was addressed was associated with research design. A research design is expected to follow the ontological, epistemological and theoretical positioning from the point of whether one believes knowledge is discovered or constructed[61]. Research questions can be answered in more than one way, so the design of research for answering research questions can take on a number of different genres. Resilience engineering, as a field of study, is relatively new, and it does not have a refined theoretical framework[62]. Traditionally engineering is the domain of science and statistics, so one would assume it can be studied using positivism[61]. However, under RE safety is not a fixed property of a system that once ‘designed in’ stays static. Rather, it is seen as an emergent property[63] and a social construction[64]. This meant that, interpretivism and social constructionism were deemed more appropriate paradigms and epistemologies in order to develop a much deeper understanding. Within this tradition if was further identified that symbolic interactionism[65, 66] provided a better theoretical framework for collecting and analysing of data, compared to pragmatism[67]]. In choosing between the two it was realized that (i) pragmatism provided a better opportunity for collecting data through mixed-method study[28-30], and (ii) getting an understanding of SWMS from construction sites was likely to produce a more practical outcome in terms of users interpretation and application SWMS. However, two ethical hurdles had to be overcome here. The first was that none of the two supervisors at the school through which the research was conducted had any previous experience with pragmatism. This lack of expertise has been previously identified as a significant issue[21]. Moreover, a mixed method study meant the researcher needed to be well-versed with both quantitative and qualitative research methods[68]. The choice made was to use symbolic interactionism and qualitative methods, mostly because the supervisors were familiar with this, and a number of successful PhDs have been previously completed using this framework. Making this ethical choice meant less time was spent on justifying to the confirmation of candidature panel why the research and theoretical framework, and the associated methods of data collection were suitable choices.The second was a consideration of how representative the data needed to be? A large criticism of case study as a methodology, among others, is that it is not representative, and the results cannot be generalized to the broader population under study[69].Addressing this meant choosing more one case study[69], and that research sites included a cross-section of the three major industry sectors of construction (commercial, residential and engineering services) were in some way represented[70]. However, going back to the research purpose suggested that achieving all of these would again be difficult especially, especially if the construction organisations failed to become give permission for their sites to be used as research labs. So it was decided that non-representative sampling[48, 71]would be used instead. Making this choice meant more efforts had to be spent on justifying to the ethics committee and panel why this approach was suitable and adequate.
5.2. Protecting Participants From Harm
- The protection of human participants involved in research can be linked with the Nuremberg Code[72, 73]. Initially aimed at clinical researchers, the code is also relevant to qualitative research because the “research may involve significant psychological costs to research participants, such as guilt, shame, fear, or embarrassment, to which the researcher may not be sensitive”[73]. Moreover, participants may feel anxious and exploited[46, 74]. Two main ways for addressing such risks include (i) ensuring those participating in the research process are provided with ample opportunity to withdraw; and (ii) ensuring the research design enables participants to be completely free in terminating their involvement at any time and for any reason[42]. A written explanation about this voluntary nature of participation must also be provided[13].In this research the approach used for protecting participants from harm was to include the following details in thePLISs: “Participation in this research is voluntary, you can refuse to participate without any explanation, and you may withdraw your consent to participate or discontinue in the research at any time.”This was reinforced by in the form of a question and response in the following manner: “Do I have to participate? No you don’t. Participating in this research is voluntary; a refusal to participate requires no explanation. You can withdraw your consent to participate and discontinue at any time until the data is processed.” These two points were repeated in the Consent Form that the informants were provided with at the start of interviews and observations, and discussed with the informants at the beginning of each interview.As a result of this approach, six (out of a total of seventy) key informant chose not participate in the interviews.
5.3. Informed Consent
- Obtaining informed consent, which is one of the cornerstones of ethical research practice[42], is based on the principles of autonomy and respect for persons[25]. In essence, “informed consent requires that prospective participants are provided with information about the project in which they are being invited to participate that is sufficiently full and accessible for their decision about whether to take part”[75]. This means ensuring that there is “a clear statement of the purposes, procedures, risks and benefits of the research project, as well as the obligations and commitments of both the participants and the researchers”[76]. The approach for ensuring informed consent in this project was as follows. Firstly, the purpose of the research was made clear in the PLISs in the following manner: “We are researching how safe work method statements are interpreted and applied to control health and safety risks in the Australian construction industry.” This was followed by an explanation of the procedures and commitments: “Should you choose to be involved in this research, you will be attending an interview with (PhD Candidate) individually or as part of a group. This interview will be semi-structured, and may take between 45 to 90 minutes. Whilst the preferred location of this interview is your normal place of work, it can be held at a location of your choice.” The time commitment highlighted in the PLISs was mostly associated with interviews, with observations this was between 3-4 hours. The statement of risks was phrased as follows: “This research study presents minimal risks for participants. You will not be asked be asked sensitive questions, and your employment will neither be enhanced nor diminished as a result of your participation (or non-participation). If participating in this research causes you any concern, you may contact a counsellor through Lifeline on (Telephone Number).”So in the PLIS was provided a justification for why the risks of participating were deemed to be low, based on a prior risk assessment. The broad benefit of the project was made clear in the following manner: “Results from this study are expected to improve the capacity of business operators and workers involved in, or closely associated with construction, to manage health and safety more effectively”. The benefit statement was derived from the Federal government’s national occupational health and safety strategy[77].The issues of participant obligations were expressed as follows: “You will first be asked to provide some background information about yourself, such as age, education, and experience in construction. These will be followed by more in-depth questions in which you will be asked to reflect and share your personal experiences in developing, revising and deploying SWMS.”Providing the information is one part of the informed consent process, the other involves getting the consent itself. The two common forms are getting a written consent or a verbal one, “obtaining consent is more than simply having a potential research participant sign a consent form; it is a process by which necessary information is communicated to the participant by the researcher”[25].The approach employed in this study involved most closely resembles one of social construction[31], which was achieved by the following. First, a copy of project summary, PLISs and consent forms were provided to the ‘gatekeepers’,with a request that these be circulated to all employees, contractors and sub-contractors of the organization. A copy was also provided to the participants before the commencement of the interviews. At this time, and after the completion of the interviews, it was re-emphasized to all the participants that their participation was purely voluntary; they were not obliged to participate or answer any questions they felt uncomfortable with, and that they were free to withdraw at any time without providing any explanation. Informants were given a choice of signing and handing back their written consent either before or at the end of the interviews and observations. The issue of getting participants to sign is a contentious one. Construction workers generally have lower levels of literacy compared to workers in other trades[78], so it may not be clearly understand what they were consenting to. In this research a two-pronged approach was utilized, by giving them information in a simple-to-understand PLIS, and alsotalking through with the participants about what this meant to them personally.
6. Ethics of Recruiting Participants
- Researchers also have ethical responsibilities when recruiting participants[79, 80]. This requires ensuring that (i) there is frank and upfront information of the goals and requirements of research, (ii) rights of participants who decline to be involved are respected, (iii) participants are not coerced into the research, (iv) individuals or groups are not exploited, and (v) there is a broad socio-demographics represented[80]. However, meeting some these requirements proved both challenging and posed ethical problems in this project.The first of these was when access had been negotiated and key informants selected, but some refused to participate in the interviews. The need to ensure participants not being harmed and free to participate and withdraw at any time meant there was one choice only; they were free to do so, and without giving any reason. However, “the principle of fidelity to science argues against such withdrawal”[81]. The ethical challenge was about balancing between adequate numbers of informants and their willingness to be involved.Another challenge, arising out of the withdrawal of participants, led the researcher to think about the reasons the informants did not want to participate. According to[81], “whenever possible, the researcher should learn from participants why they wish to withdraw. This information is important because it might reveal a problem in a research procedure that needs to be addressed. Sometimes these conversations can reveal underlying reasons about why participant’s wishing to withdraw are for a reason that the researcher can address. In such cases, the participant may choose to remain in the research”. Was it because of the researcher’s previous role as in inspector for the regulator? Was it because of his colour? Was it something they themselves had been coerced into? Or was it because of the types of questions they were being asked? No reasons for refusing to participate in the research were sought from the first four informants who chose to withdraw. In doing so the researcher was abiding by one of the key principles associated with protection of harm; participants were free to withdraw at any stage without any reason. However, after the first four had had done so, a different tact was applied and the next two were asked if they wanted to share with the researcher why they wanted to withdraw. One informant was not against the participation itself, he was a subcontractor, he had been informed and he was being paid for the time he spent with me, so there were no issues there. What he was not comfortable with was with the tape-recording of his interview, he believed this could be misused. He was happy to be interviewed provided it was not tape-recorded. His colleague, who was an apprentice, basically followed the stance of his boss.The way this was dealt with this particular concern was to document the responses from these two informants as diary notes, which were later written up as ‘re-constructed notes of focus group.’ In this instance the researcher’s previous experience as an inspector, where he had collected data through safety management system audits and investigations of workplace incidents helped greatly. So by asking the participants the reasons why they wanted to participate enabled the researcher to address some of their concerns as part of the data collection strategy.
7. Discussion
- Ethics is concerned with how people behave when they are faced with a conflict between two or more principles to which they subscribe[82], and it is an important issue that both novice and experienced health and safety researchers would need to address as part of their research journey. The guidelines established by UHREC are a useful starting point; however, there are not a panacea for successful conduct of qualitative research in health and safety in construction settings. What become important are the researchers’abilities to deal with issues as they arise across the research journey. In this research the absence of prior expertise in the school meant disregarding pragmatism in favour of interpretivism as an epistemological stance, and disregarding mixed methods in favour of qualitative methods. The moral judgement made here was based on the researchers not being disadvantaged due to lack of expertise in the school. The limitations of drawing conclusions from case study research meant choosing more than one case, and the issue of representation meant a closer consideration of possible research sites. Because it may not be possible to do representative sampling, it is then necessary to justify the choice of sampling utilized, and why the approach being used was likely to produce authentic results. Protecting participants from harm means they should be free to withdraw from the research project, however doing so means a rethink of whether one can get adequate data. Seeking informed consent can be particularly challenging if the participants are required to sign off on a form, so consideration needs to be given to extra efforts in explaining to the participants what it is they are consenting to. Getting away from written consent to oral consent may be a way forward.The ability to get construction organizations as possible research sites was perhaps one of the most difficult ones to negotiate. In this research it was possible largely through the use of colleagues the researcher had previously worked for. To deal with the ethical issue of ‘regulator coercion’ the strategy utilized was to couch the researcher’s previous experience in the PLIS. This may not be an issue if the researcher has not necessarily worked with the organization before, or has had previously dealt with the organisations in another capacity, especially if this involved enforcement. Some of the ways in which this has been suggested to be overcome include (i) allowing sufficient time for access and entry, (ii) using friends and relatives wherever possible, and (iii) using non-threatening language when explaining nature and purpose[83].Once access has been obtained additional ethical issues may arise when one is seeking to recruit informants. Here, frank and upfront information of the goals and requirements of research, right of participants to withdraw from research at any stage, reducing and/or limiting coercion, and ensuring broad representation of participants are key considerations. While addressing all of these may create additional challenges for research, frank and open communication with the participants was necessary to allay some of the concerns and fears associated with participating in research. Interviewees with lower than normal levels of literacy skills, as is common in the construction industry, can also interpret questions differently, and there is a need to unify the interview strategy in some way. Moreover, dealing positively with respondent’s reservations with respect to time and confidentiality, even offering a report of findings can become important[83].This paper has examined ethical issues associated with planning and recruitment, in particular those that need to be considered as part of research design. Some of the key principles at these stages include protecting participants from harm, informed consent and free will to withdraw. They have to be balanced against the need to ensure a unified interview strategy, adequate numbers for participation, some of which may not be really clear at the planning and recruitment stages.
8. Conclusions
- In order to ensure the ethical conduct of research, novice qualitative researchers and those intending to do such research in health and safety in construction settings can use the guidelines provided by UHRECs as a starting point. However, as has been discussed in this article, it is not a panacea for successful conduct of research. The approach used is this particular study discussed actual ways in which ethical issues such as protection of harm, informed consent, freedom from participation at research design, planning and recruitment were addressed. Ethical issues pervade the entire research process, including data collection, analysis and reporting, and how these were addressed in this study will be the subject of a future paper. It is hoped this article will inspire other researchers to reflect and write about their experiences on the actual conduct of ethical research.
ACKNOWLEDGMENTS
- I would like to thank the anonymous referees for their helpful comments and assistance which helped in refining this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML