-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2017; 7(1): 25-34
doi:10.5923/j.scit.20170701.03

Experimental Verification of Dynamic Modelling of Nitrogen Adsorption on Zeolite 13X with VSA Process
Abolfazl Karimi1, Peyman Emrani1, Marjan Haghayegh2
1School of chemical Engineering, University of Tehran, Tehran, Iran
2Young Researchers and Elites club, Science and Research Branch, Islamic Azad University, Tehran, Iran
Correspondence to: Abolfazl Karimi, School of chemical Engineering, University of Tehran, Tehran, Iran.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

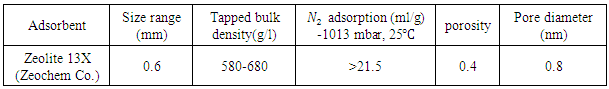
The four-step vacuum swing adsorption process applying zeolite 13X is studied in order to separate oxygen from air. Effects of the duration of vacuum stage, pressure drop and NMP solvent as a coat of the adsorbent are evaluated. An adsorption model is utilized for isothermal adsorption system by different mechanisms containing local equilibrium and solid diffusion model. Linear isotherm can describe the adsorption process, appropriately. Moreover, linear driving force, LDF, is used as the mass transfer approximation, in solid diffusion model. By this approximation and finite difference solution, ODE equations are solved by Runge-Kutta method using MATLAB. Solid diffusion model utilizing one fitting parameter predicts the breakthrough curve properly more compatible than another theory. The appropriate solid diffusion coefficient utilized as the fitting parameter is 10-10 m2/s. The model accuracy is evaluated, and the error values of equilibrium and solid diffusion model from experimental data are reported 11.7, 1.17, respectively.
Keywords: Vacuum swing adsorption, Zeolite 13X, Oxygen, Nitrogen, Mathematical model
Cite this paper: Abolfazl Karimi, Peyman Emrani, Marjan Haghayegh, Experimental Verification of Dynamic Modelling of Nitrogen Adsorption on Zeolite 13X with VSA Process, Science and Technology, Vol. 7 No. 1, 2017, pp. 25-34. doi: 10.5923/j.scit.20170701.03.
Article Outline
1. Introduction
- Oxygen is used in the various aspects such as metallurgical industry, glass-making and ceramic industry, and wastewater treatment [1]. Vacuum/ pressure swing adsorption process (VSA/PSA) is the developed operation to separate and purify gasses, which is based on adsorption ability and selectivity of an adsorbent [5, 6]. In 1970, this method was initially utilized to generate oxygen from air. This technology has advantages compared with various procedures used to separate of air and manufacture oxygen such as cryogenic distillation, membrane and chemical absorption method [2]. In other words, VSA/PSA process is a safe and automatic method, which requires small investment with low energy requirement that would be put in/exit from the service easily [1]. The development of highly selective adsorbents such as LicaX and LiAgX facilitates the generating oxygen up to 200 tons/day by VSA process, which is competitive with cryogenic distillation [2]. Also, VSA process is more economic to produce of over 15 ton/day oxygen in comparison with PSA process [1, 2].In VSA process, adsorption and desorption pressure, and the adsorbent capacity are key factors to separate of air, efficiently. The optimal vacuum pressure and time play the critical role to reduce the cost of equipment and energy. For air separation, most industries operate VSA process in the ambient temperature, adsorption pressure of 1.1 to 1.8 atm and desorption pressure of 0.05 to 0.3 atm. Moreover, choosing the regeneration method depends on the adsorbent, adsorption ability and operating condition of the unit [2]. Apart from the experimental investigations, mathematical modeling of VSA process is widely utilized to determine the optimum conditions. Moreover, modeling methods can be useful for process development and scale-up, and provide conceptual procedures to estimate some parameters which are necessary for process design purposes [1, 5, 9].Linear driving force (LDF) has been applied as a suitable approximation to describe the rate coefficient for extra particle and intra particle mass transfer controlling. Also, intra particle mass transfer can be predicted by four main region: local equilibrium, pore diffusion, solid diffusion, and combined pore and solid diffusion. By these approximation procedures and finite difference method, solid concentration is obtained by solving Ordinary Differential Equation (ODE) equations instead of Partial Differential Equation(PDE) [10, 11]. LDF approximation with linear equilibrium has been utilized to model the PSA process [12, 13]. Pore diffusion model with assumption of linear adsorption isotherm and axial dispersion has been used to simulate non isothermal process [14, 15]. Hwang and Lee have also applied LDF model and binary Langmuir isotherm to predict the experimental breakthrough behaviors of CO and CO2 on activated carbon in isothermal condition with axial dispersion, plug flow and no radial gradient of concentration [16]. Moreover, this model has been employed for isothermal PSA process using zeolite in a column with plug flow and no radial gradient of concentration [17, 18]. LDF model with Langmuir isotherm has been studied to predict breakthrough in the fixed bed with axial dispersion and plug flow [19]. A non-isothermal dynamic model using LDF model and the Langmuir-Freundlich isotherm has been applied for VSA process of removing N2 from air using zeolite 13X and 10X. In this study zeolite 13X has produced product with higher purity [2]. Budner et al. has generated oxygen by VSA process using CaX zeolite, and provided the non-equilibrium model [1]. The main goal of the present work is the investigation of the operating condition of VSA process which is used to adsorb nitrogen from air by zeolite 13X. For this purpose, the experiments operated in consecutive processes are designed to determine the adsorption time and vacuum required, and the appropriate result is presented in this work. Also, two models of VSA process including equilibrium and solid diffusion model are studied. In order to evaluate the model validity and determine the appropriate model, the results from the mathematical model are compared with experimental data.
2. Experiment
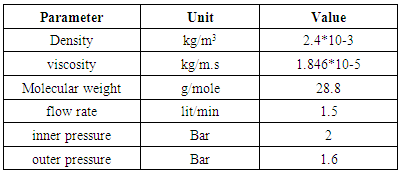
- a) Vacuum-swing adsorption system:The schematic of the vacuum-swing adsorption apparatus is shown in figure 1. VSA process consists of four stages: pressurize, adsorption, depressurize, and vacuum operation. Adsorption system designed as bench scale includes an adsorption column with pipes, valves, rotameter, oxygen sensor (SENKO-SS1118), vacuum pump (VALUE- VE115), hardware for Data acquisition and software package of SENKO Co. to convert and monitor results. The length and inner diameter of adsorption column made of stainless steel pipe are 0.2 and 0.1 m, respectively. Two perforated plates with mash consisting of thin hole are installed on both ends of column to fix adsorbent. Oxygen sensor and its transmitter which are located in a handmade sheath can measure the concentration with response time of 15 to 20 seconds. Oxygen concentration versus sampling time is stored as HEX format, and it is converted to decimal numbers by coding in MATLAB. Sampling time is adjustable, and it is considered 1 second which is appropriate regarding to adsorption rate. Moreover, since oxygen sensor should not be under gas pressure, a needle valve is utilized after the adsorbent column and in front of the sensor.
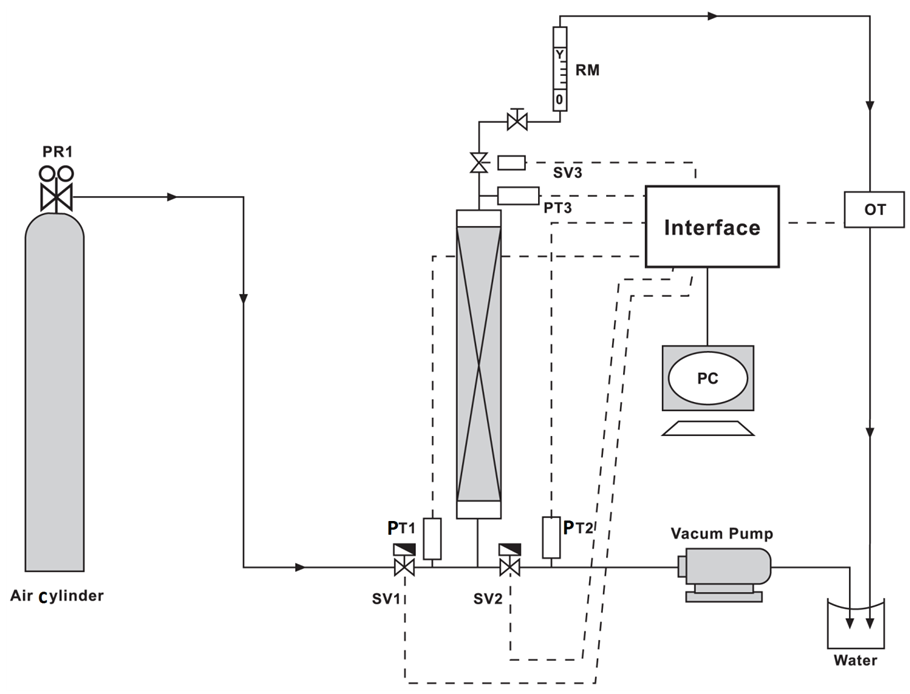 | Figure 1. Schematic of VSA apparatus, PR = pressure regulator, PT = pressure transducer, RM=Rotameter, OT= oxygen transducer, V=valve, SV=switch valve |
|
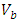 is filled with the adsorbent. By weighing this cylinder containing adsorbent
is filled with the adsorbent. By weighing this cylinder containing adsorbent 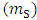 and one particle of zeolite, the number of particles is determined in this volume, so the volume of all particles is calculated. By subtracting of this volume from
and one particle of zeolite, the number of particles is determined in this volume, so the volume of all particles is calculated. By subtracting of this volume from 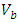 , the volume of empty space is calculated as follow.
, the volume of empty space is calculated as follow.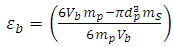 | (1) |
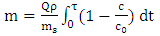 | (2) |
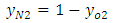 | (3) |
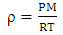 | (4) |
3. Mathematical Modeling
- Mathematical model is required to perceive the behavior of the adsorption bed during the VSA process. This model is based on the following assumptions: (1) The process is isothermal.(2) The particles are spherical and they are packed uniformly into the fixed bed with homogeneous porosity.(3) Equilibrium equations of nitrogen and oxygen components are represented by binary Henry isotherm.(4) The gas phase behaves as an ideal gas.(5) The flow pattern is described by the axially dispersion flow.(6) Radial concentration gradient is negligible.The component mass balance for the gas phase in the adsorption bed is derived by the following equation [8]:
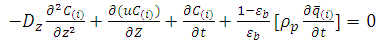 | (5) |
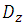 , is obtained as follow [23]:
, is obtained as follow [23]: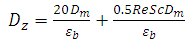 | (6) |
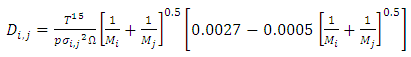 | (7) |
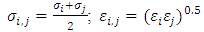 | (8) |
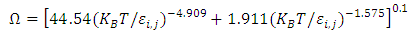 | (9) |
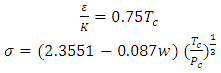 | (10) |
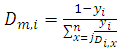 | (11) |
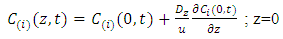 | (12) |
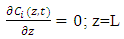 | (13) |
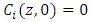 | (14) |
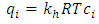 | (15) |
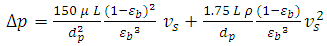 | (16) |
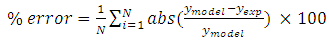 | (17) |
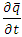 in equation (5) is replaced by
in equation (5) is replaced by 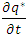 .
. 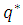 is the adsorbed equilibrium concentration in solid phase, which is determined by Henry isotherm as follow [10, 11]:
is the adsorbed equilibrium concentration in solid phase, which is determined by Henry isotherm as follow [10, 11]: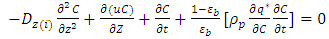 | (18) |
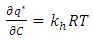 | (19) |
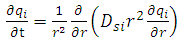 | (20) |
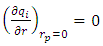 | (21) |
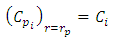 | (22) |
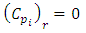 | (23) |
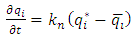 | (24) |
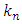 is calculated by equations (23-24) considering R=1 for linear isotherm [8].
is calculated by equations (23-24) considering R=1 for linear isotherm [8].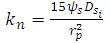 | (25) |
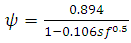 | (26) |
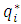 is estimated by Henry isotherm, and the average amount of adsorption
is estimated by Henry isotherm, and the average amount of adsorption 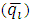 is calculated by solving the set of differential mass balance equations of particle. Finally the equation of gas phase in the bed (Eq. 5) and particle phase (Eq. 20) are solved simultaneously, according to the boundary conditions of the particle.
is calculated by solving the set of differential mass balance equations of particle. Finally the equation of gas phase in the bed (Eq. 5) and particle phase (Eq. 20) are solved simultaneously, according to the boundary conditions of the particle.4. Result and Discussion
- Figure 2 represents experimental oxygen composition data versus time. The characteristics of air as feed is stated in table 2. According to figure 2, the maximum concentration of oxygen is produced during 0 to 100 seconds because the sites of adsorbent contain no adsorbate at the beginning of the process, so it has the comprehensive potential to adsorb nitrogen. By passing air, the adsorbent becomes saturated, and the adsorption rate decreases between 100 to 280 seconds.
 | Figure 2. Temporal profiles of effluent oxygen composition |
|
|
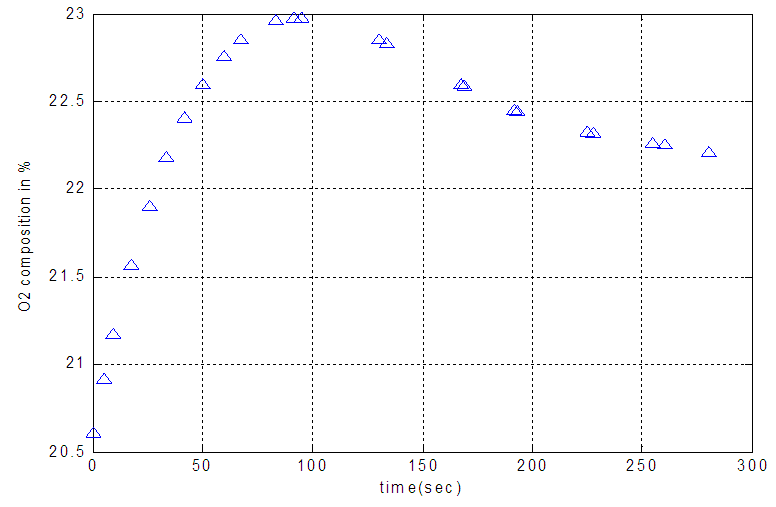
 | Figure 3. Temporal profiles of effluent oxygen composition in different time of vacuum pressure (560 torr) |
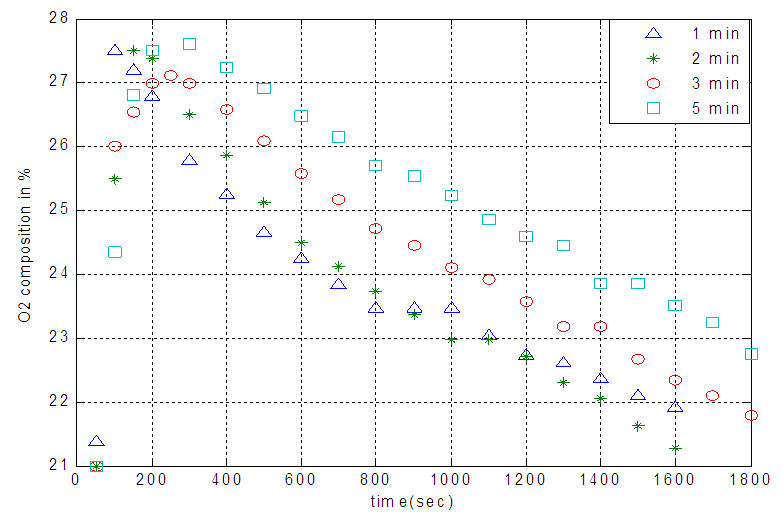
 | Figure 4. Temporal profiles of effluent oxygen composition of zeolite 13X covered by the various amount of NMP |
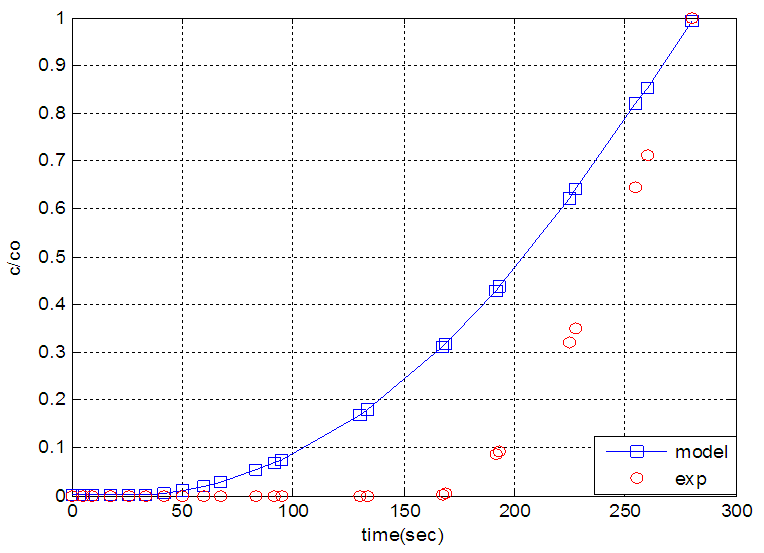 | Figure 5. The experimental breakthrough curve of nitrogen modeled by equilibrium theory |
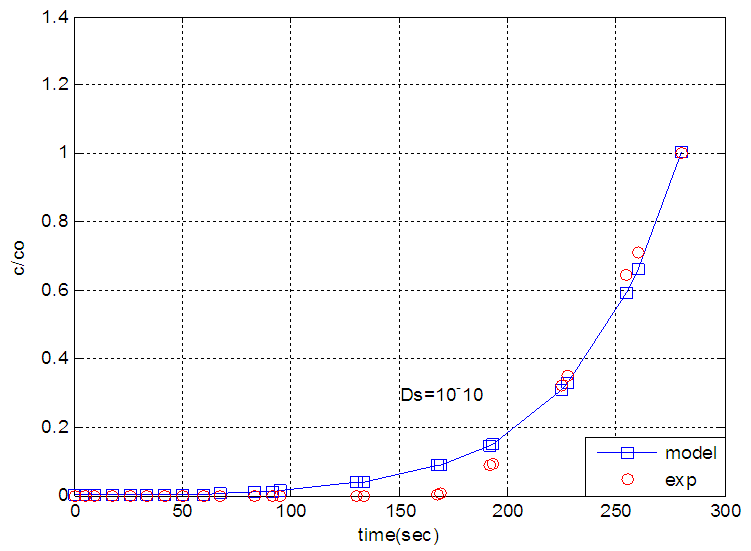 | Figure 6. The experimental breakthrough curve of nitrogen modeled by solid diffusion theory |
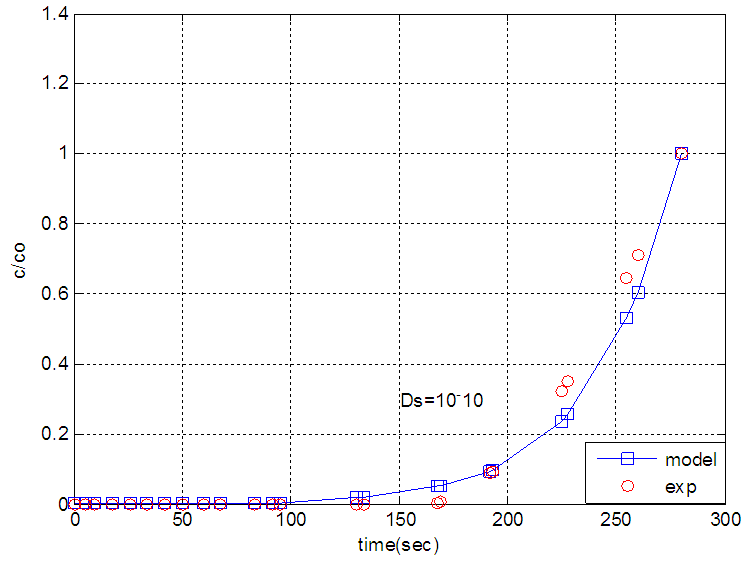 | Figure 7. The experimental breakthrough curve of nitrogen modeled by solid diffusion theory without dispersion |
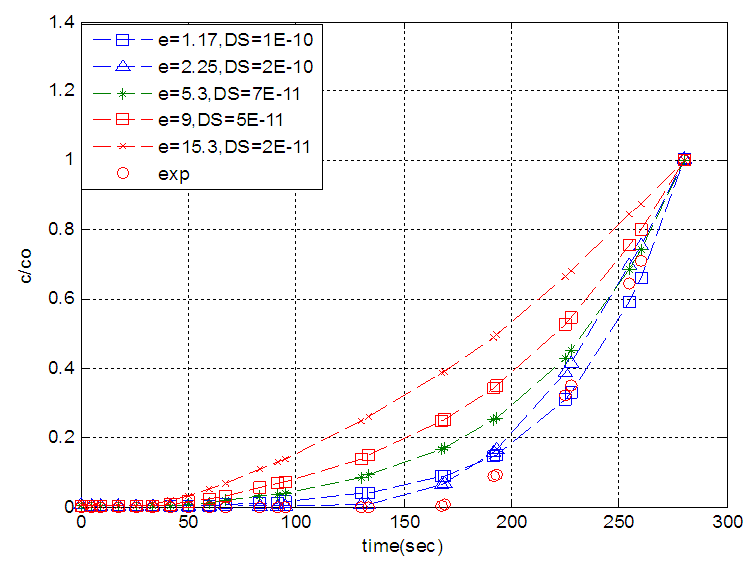 | Figure 8. The effect of zeolite diffusivity on solid diffusion model |
5. Conclusions
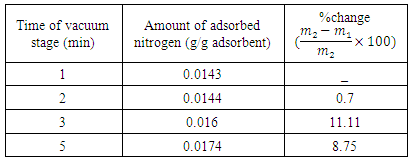
- Vacuum swing adsorption using zeolite 13X is carried out in order to produce oxygen by separating air. It is obtained that VSA process is much more appropriate and applicable than PSA. According to study the effect of vacuum stage on adsorbing nitrogen, the time of 3 minutes is suitable to regenerate the adsorbent. Moreover, it is observed that coating the zeolite by NMP solvent has the detrimental effects on the structure of the adsorbent. Hence, the low amount of NMP decreases the adsorption, and its high amount deactivates the zeolite. Also, the pressure drop during the experiments is compared with Ergun equation, which has 11% error. The mass balance of the adsorption bed with isothermal process, axially dispersion flow and Henry adsorption isotherm is applied to study the adsorption of nitrogen. This model is investigated for two cases containing local equilibrium, and solid diffusion model. Solid diffusion model with LDF approximation is used successfully to correlate the adsorbed nitrogen versus time. The modeling result is improved by using adsorbent diffusion coefficient as a fitting parameter.% error values are 11.7% for equilibrium model and 1.17% for solid diffusion model with one fitting parameter.
ACKNOWLEDGEMENTS
- Authors of this work wish to appreciate Dr. Fatemeh Zabihi and Dr. Ali Farzi for their attendance and Alireza Salehpour for his technical support and consultation.
Abbreviation

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML