-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2016; 6(3): 76-88
doi:10.5923/j.scit.20160603.03

Antioxidant Activity of Apple Peels Bioactive Molecules Extractives
Najlaa K. Issa1, Rihan S. Abdul Jabar2, Yousif H. Hammo3, Ibtisam M. Kamal4
1Department of Chemistry, Faculty of Science, University of Duhok, Kurdistan Region of Iraq
2Department of Chemistry, Faculty of Science, Zakho University, Kurdistan Region of Iraq
3Department of Hort., Faculty of Agr. and Forestry, Duhok University, Kurdistan Region of Iraq
4Department of Chemical Engineering, Faculty of Engineering, Soran University, Kurdistan Region of Iraq
Correspondence to: Ibtisam M. Kamal, Department of Chemical Engineering, Faculty of Engineering, Soran University, Kurdistan Region of Iraq.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the present work apple peel extracts of (Total Phenols, Flavonoids, and Anthocyanin) compounds from the fruits cultivated in Kurdistan Region of Iraq (BarwariBala mountains in Duhok governerate) are prepared. The three extracts are evaluated concerning the yield and the antioxidant activity of the bioactive compounds by four methods (β-Carotene- linoleic acid assay, reducing power assay, scavenging of hydrogen peroxide, and total antioxidant capacity). The factors affecting antioxidant activity including temperature, pH and incubation time were optimized using Response Surface Methodology. The effect of storage time on antioxidant activity was also studied. The results confirmed that the yield of total phenols were 23.33%, about 31.20 % of the total phenols were flavonoids, while anthocyanin compounds formed 9.76% (g/g raw material). The three extracts showed antioxidant activities of different extents. Response surface analysis results revealed that increasing the incubation time and temperature resulted in decrease antioxidant capacity; however, temperature seemed of top most significant compared to incubation time. The antioxidant capacity of the extracts showed gradual decrease with increasing pH up to pH= 9, followed by an increase towards high pH values. The antioxidant capacity of all extracts were gradually reduced with increasing storage time. The antioxidant capacity of anthocyanin extract was slightly affected by storage time compared to alcoholic and flavonoids extracts. The overall results confirm that the high content of phenolic compounds and antioxidant activity of apple peels indicates that they should be regarded as source of antioxidants.
Keywords: Antioxidant activity, Apple peel, Response Surface Methodology
Cite this paper: Najlaa K. Issa, Rihan S. Abdul Jabar, Yousif H. Hammo, Ibtisam M. Kamal, Antioxidant Activity of Apple Peels Bioactive Molecules Extractives, Science and Technology, Vol. 6 No. 3, 2016, pp. 76-88. doi: 10.5923/j.scit.20160603.03.
Article Outline
1. Introduction
- A characteristic of higher plants is the production of a wide variety of natural products, called secondary metabolites; the majority of them have economical and industrial importance drugs, flavor and fragrance, dye and pigments, pesticides, and food additives. Research on natural products with potential health benefits represents an area of great interest in which fruits had been the most important source.Apple (Malus domestica) of the rose family (Rosaceae) is a delicious fruit and is the fourth most widely produced fruit worldwide (Konarska, 2013). Apple has got high medicinal value; the pulp, the seeds and the peels possess medicinal property (Balasuriya and Rupasinghe, 2012; Ismail et al., 2012; Otakar et al., 2012; Thilakarathna, and Rupasinghe, 2013). There is a great abundance of literature data concerning the medical benefits of apple are such as antioxidative, anticancer, and antimutagenic efficacy (Boyer and Liu, 2004; Rapasingle et al., 2011; Giomaro et al., 2014 ). Moreover, as compared with some other core fruit species (e.g., pears), apples contain less energy and show a high content of minerals, pectins and vitamin C (Rapasingle et al., 2011).Apple peel as by-product normally is directly disposed as waste. The results from previous studies showed that the peel has high content of phenolic compounds, antioxidant activity, and antiproliferative activity (Al-Zoreky 2009; Balasuriya and Rupasinghe 2012; Woife et al., 2003; Huber and Rupasinghe; 2009; He and Liu, 2008; Massini et al., 2013). Furthermore, Many studies revealed that independently of apple varieties and other circumstances, peel contains more phenolics than the flesh (Kalinowska et al., 2014; Drogoudi et al., 2008; Kalinowska et al., 2012). In general, the antioxidants are beneficial because they neutralize free radicals that can damage cells and tissues. The most widely used synthetic antioxidants in food (butylatedhydroxytoluene BHT, butylatedhydroxyanisole BHA, and tertiary butyl hydroquinone TBHQ) have been suspected to cause or promote negative health effects (Pokorny, 2007; Yoshihara et al., 2010). For this reason there is a growing interest in studies of natural additives as potential antioxidants for several industrial applications, mainly concerning the cosmetic, and the food preservation and formulation (Mendiola et al., 2010).The antioxidants level is significantly influenced by the apparatuses used in the extraction, the nature of the extraction solvent, extraction time and temperature, as well as the interaction between these factors. However, it was reported that solvent extracting power is the most important factor affecting antioxidant capacity (Jin et al., 2015; Dorta et al., 2013) followed by the apparatuses that are used in the extraction ( Zang et al., 2014).On the other hand, the antioxidant activities is highly affected by storage conditions. Most of the studies carried out in concern confirmed that antioxidant activity decreases during storage. The reason may be attributed to dilution of antioxidant components by increased moisture and also to possible oxidation of antioxidant components (Dar and Nayik, 2016). The exposure to heat and light during storage could change or degrade the structure of the antioxidants, resulting in marked changes in their affinity (Fracassetti et al., 2013; Del-Toro-Sánchez et al., 2015).A number of studies have been carried out on the phenolic compounds and antioxidant In Kurdistan region of Iraq, there are many members of the genus Malus that are used in diet, thus, the main aim of the work described here is to focus on the fruit waste; the peel of typical native apple cultivars and to determine the basic chemical and biological characteristics which are incompletely known such as the activities of the different bioactive molecules in order to improve knowledge of apple peels as raw material for antioxidant production, and to contribute to their impact on the management of a variety of clinical conditions and maintenance of health.
2. Experimental
2.1. Raw Material
- Apple fruit was collected from Kurdistan Region of Iraq (BarwariBala Duhok mountains). The fruits were peeled and the fresh peels were extracted after minced to very fine pieces.
2.2. Chemicals and Reagents
- All the used solvents and chemicals were of analytical grade produced from Sigma Aldrich.
2.3. Preparation of Plant Extracts
2.3.1. Extraction of total Phenol Content (alcoholic extract)
- The alcoholic extract was prepared following the procedure of (Laleh et al., 2006; Banso, 2009).Thirty g of the fresh peels were mixed with 200 ml (70%) ethanol using electrical blender and the mixture was shaken in thermostatic water bath at 40°C for 4 h. The residue was removed by filtration using filter paper (Whattman No. 40) and the filtrate was concentrated by evaporation using rotary evaporator to afford (7.00 g ) of extract.
2.3.2. Flavonoids Extract
- The flavonoid extract was prepared following literature procedure (Peach et al., 1955; Harborne, 1984; Andersen and Markham, 2006). Five g of alcoholic extract were dissolved in (100 ml) distilled water then 125 ml of (1%) aqueous lead acetate was added with stirring for 5 min. The solution was left for 2 h to complete the precipitation. The solution was filtered using filter (Whattman No. 40) and the filtrate was concentrated by rotary evaporator. The resulted semi-solid material was then dissolved in absolute methanol. Fifty ml of (1%) methanolic lead acetate was added with stirring for few min and the solution was left for 2 h for precipitation completion. The mixture was filtrated using Buchner funnel and the precipitate was washed once with 10 ml distilled water, then with 10 ml absolute methanol and finally washed for 3 times with 10 ml ethyl acetate.The precipitate was left to dry in dark at room temperature. The dry precipitate was dissolved in [25 ml acetone + 5 ml (2M HCl)] with shaking, and then left till the complete precipitation of white precipitate. The supernatant was separated and evaporated under vacuum at 40°C to afford (1.56 g) extract which referred as flavonoid extract.
2.3.3. Anthocyanin Extract
- The anthocyanin extract was prepared following the procedure mentioned by (Harborne, 1984; Schofs, 2004; Andersen and Markham, 2006). Twenty five g of the fresh peels were mixed for 5 min with 50 ml of acidified methanol (1% HCl in methanol) using electrical blender. The mixture was transferred to amber conical flask and placed in an ice bath and stirred for 12 h. The mixture was filtrated by Buchner funnel and the filtrate was collected and placed in dark at room temperature till dryness. The dry material was washed twice with (25 ml) acidified methanol and filtered to obtain (2.44 g) extract which referred as anthocyanin extract.
2.4. Antioxidant Activity of the Extracts
- The antioxidant activity for the 3 extracts was evaluated by 4 methods:
2.4.1. β-Carotene- Linoleic Acid Assay
- In β -carotene-linoleic acid assay, the antioxidant activity (AA) of the 3 apple peel extracts were compared with antioxidant activity of a synthetic antioxidants (α-tocopherol) during subjected to thermal autoxidation at 50°C following the procedure of (Marco, 1968). The mechanism of bleaching of β-carotene is a free-radical-mediated phenomenon resulting from the hydroperoxides formed from linolic acid. β-carotene, in this model system, undergoes rapid discoloration in the absence of an antioxidant. The linoleic acid free radicals, formed upon the abstraction of hydrogen atom from one of its diallylic methylene groups, attacks the highly unsaturated β-carotene molecules. As β-carotene molecules lose their double bonds by oxidation, the compound loses its chromophore and characteristic orange color, which can be monitored spectrophotometrically.The presence of different extracts can hinder the extent of β-carotene-bleaching by neutralizing the linoleate-free radical and other free radicals formed in the system. The values of AA are tabulated in Table (2), and plotted as shown in Figure (1).
2.4.2. Reducing Power Assay
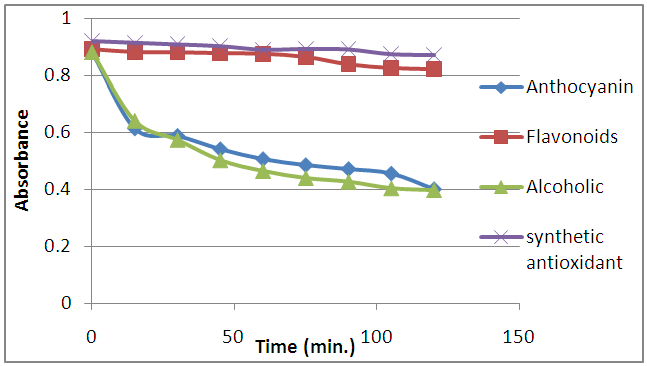
- The ability of alcoholic, flavonoids, and anthocyanin extracts of apple peels to reduce iron (III) to iron (II) was determined according to the procedure of (Oyaizu, 1988; Yildirim et al., 2001; Tsasi et al., 2006; Su et al., 2009) and compared to that of ascorbic acid, which is known to be a strong reducing agent. The % reduction (R.P. %) of the samples was plotted against concentration as shown in Figure (2).
2.4.3. Scavenging of Hydrogen Peroxide
- The ability of alcoholic, flavonoids, and anthocyanin extracts of apple peels to scavenge H2O2 was determined following the methods of (Ruch et al., 1989; Oktay et al., 2003) using the calibration curve between concentration and absorbance at 230 nm of standard ascorbic acid. The results of the test are shown in Table (3).
2.4.4. Total Antioxidant Capacity
- The test is based on the reduction of Mo (VI) to Mo (V) by the samples of alcoholic, flavonoids, and anthocyanin extracts of apple peels. The antioxidant capacity of the extracts was evaluated following a literature method of (Prieto et al., 1999 and Deloueeet al., 2007) and expressed as micromoles of α-tocopherol equivalent per milliliter of extracts using the calibration curve of α-tocopherolat 695 nm. The concentration was determined from Beer’s Law using extinction coefficient of (4 x 103 M-1 cm-1). The results of the test are shown Figure (3).
3. The Impact of Temperature, pH, and Heating Time on the Antioxidant Activity
3.1. Statistical Analysis Using Response Surface Methodology (RSM)
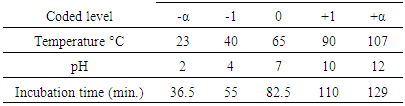
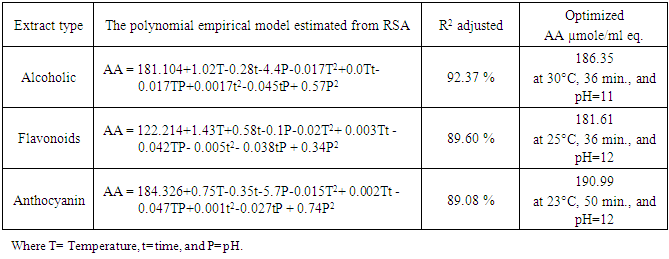
- Response surface methodology (RSM) in conjunction with central composite rotatable design (CCRD) was performed in the present study after preliminary experiments. The RSM was used for optimization the impact of temperature, pH, and heating time on antioxidant activity for the 3 extracts.RSM was introduced by Box and Wilson in 1951 (Box and Wilson, 1992). The methodology is commonly used for the experimental design (Elaydi et al., 2014). The RSM normally allow identifying the effects of operating parameters (independent variables Xi) on different responses Yj (dependent variables).The statistical analysis procedure of statgraphics plus for windows software (5.1 version) for experimental design and data treatment was used and a central composite design consisting of 18 experimental runs with 4 replicates at the center point was established. The mathematical empirical model applies in this study was:
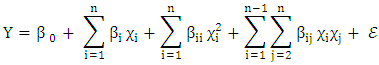 where Y: is the response or dependent variable;
where Y: is the response or dependent variable; 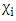 and
and 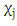 : the independent variables; i and j: the indices;
: the independent variables; i and j: the indices; 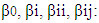 the regression coefficients; and
the regression coefficients; and 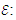 is the random error of the factors.The analyses of variance (ANOVA) were used to determine significant differences between the independent variables (p<0.05). Pareto chart was used to identify the impact of variables on various responses. The vertical line in the Pareto chart determines the effects that are statistically significant at 95% as confidence level. Surface methodology plots were used to optimize the various responses. The levels of independent variables used in experimental design are listed in Table 1 which shows the independent variables and their levels. α the (axial distance)
is the random error of the factors.The analyses of variance (ANOVA) were used to determine significant differences between the independent variables (p<0.05). Pareto chart was used to identify the impact of variables on various responses. The vertical line in the Pareto chart determines the effects that are statistically significant at 95% as confidence level. Surface methodology plots were used to optimize the various responses. The levels of independent variables used in experimental design are listed in Table 1 which shows the independent variables and their levels. α the (axial distance) 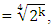 k is the number of orthogonal design variables (in the present case, k=3).
k is the number of orthogonal design variables (in the present case, k=3).
|
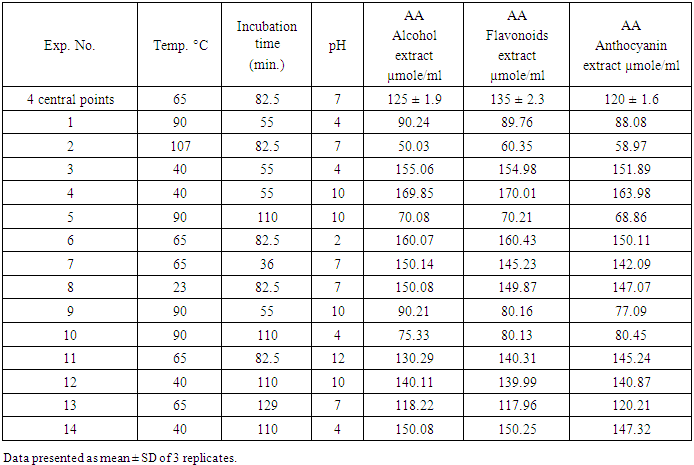
3.2. Determination of the Dependent Variable (The Antioxidant Activity: AA)
- The residual antioxidant capacity of alcoholic, flavonoids, and anthocyanin extracts of apple peels were determined for the extracts treated according to the experiments established by RSM. The response values are listed in Table 2.
|
4. Results and Discussion
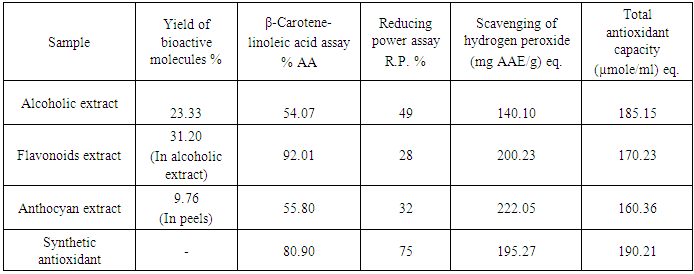
- Phenolic compounds are one class of antioxidant agents which act as good proton donors resulting in free radical terminators and contributed to the antioxidant activities of plant (Garzn and Wrolstad, 2009). Flavonoids are groups of polyphenolic compounds, which exhibit several biological effects such as anti-inflammatory, anti-hepatotoxic, antiulcer, anti-allergic, anti-viral, and anticancer activities.They also act as inhibitors enzymes such as reducates and xanthine oxidase. They are capable of effectively scavenging the reactive oxygen species because of their phenolic hydroxyl groups and are potent antioxidant (Su et al., 2009). Escarpa et al., 1998 and Wolfe et al., 2003 and other researchers reported that apple peels possess high contents of phenolic compounds compared to other edible parts of the apple. The fact suggests that apple peels may possess more bioactivity than the flesh. It was also reported that the anthocyanin content of apple peels is related to their appearance. The red color of apple peels is due to the presence of cyanidin 3-galactoside (Awad et al., 2000).The yield of the bioactive molecules, and the evaluation results of AA of the 3 extracts and the synthetic antioxidant samples of identical concentrations tested by different methods are shown in Table 3.
|
 | Figure 1. Antioxidant activity of alcoholic, flavonoids, and anthocyanin extracts of apple peels compared to α-tocopherol |
 | Figure 2. Reducing power of alcoholic, flavonoids, and anthocyanin extract of apple peels as a strong reducing agent compared to Ascorbic acid |
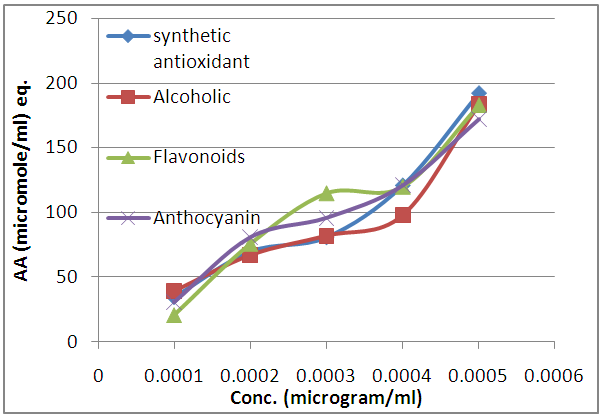 | Figure 3. Total antioxidant capacity of alcoholic, flavonoids, and anthocyanin extracts of apple peels compared to the synthetic antioxidant |
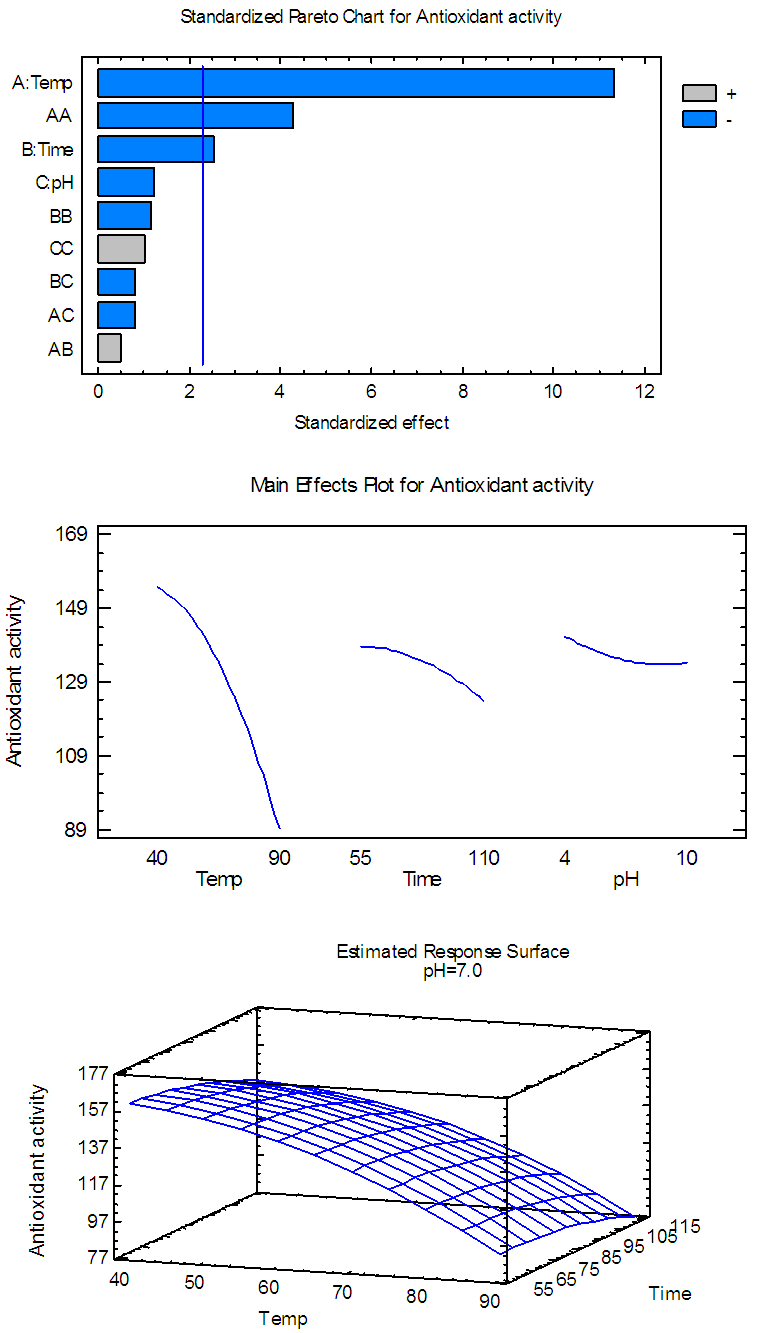 | Figure 4(a). Pareto chart, general trends, and response surface of the effects of temp., time and pH on AA for flavonoids extract |
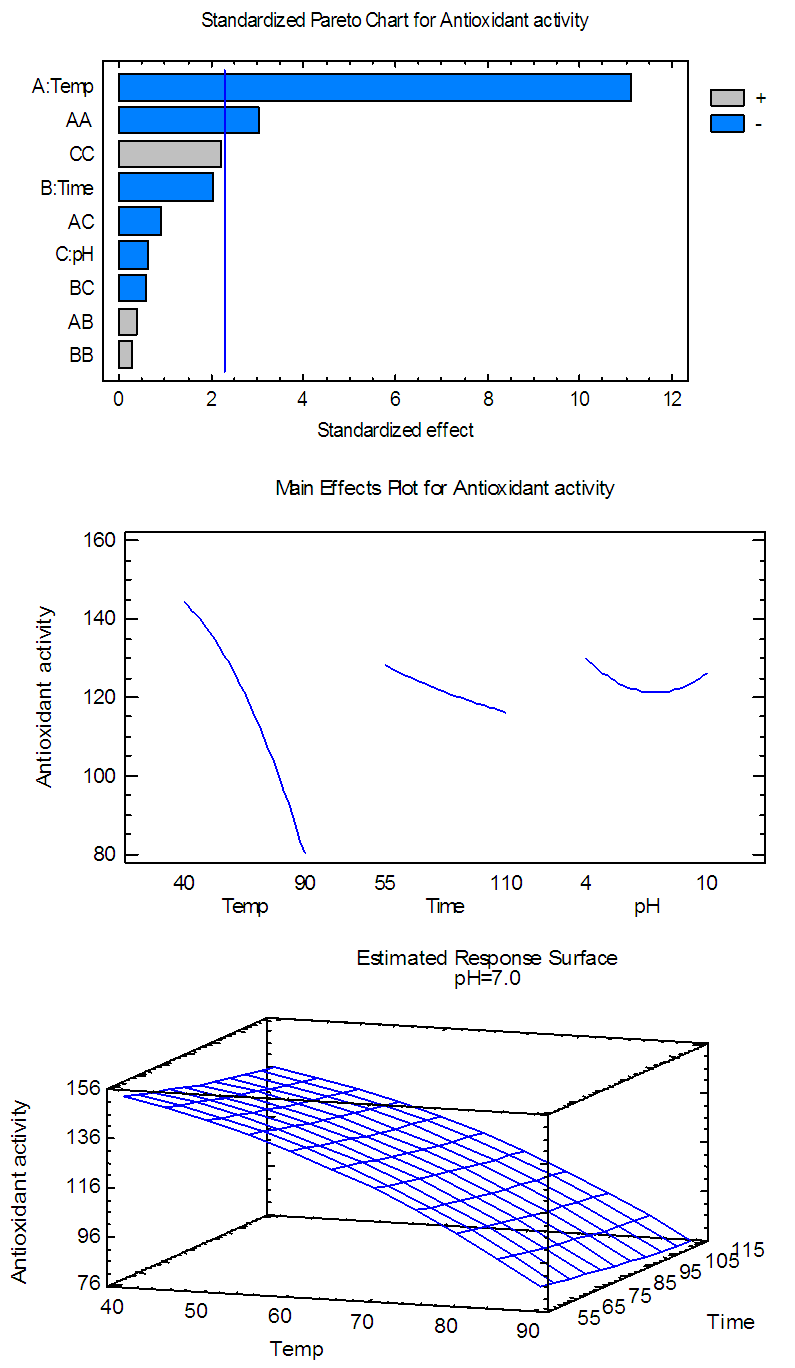 | Figure 4(b). Pareto chart, general trends, and response surface of the effects of temp., time and pH on AA for anthocyanin extract |
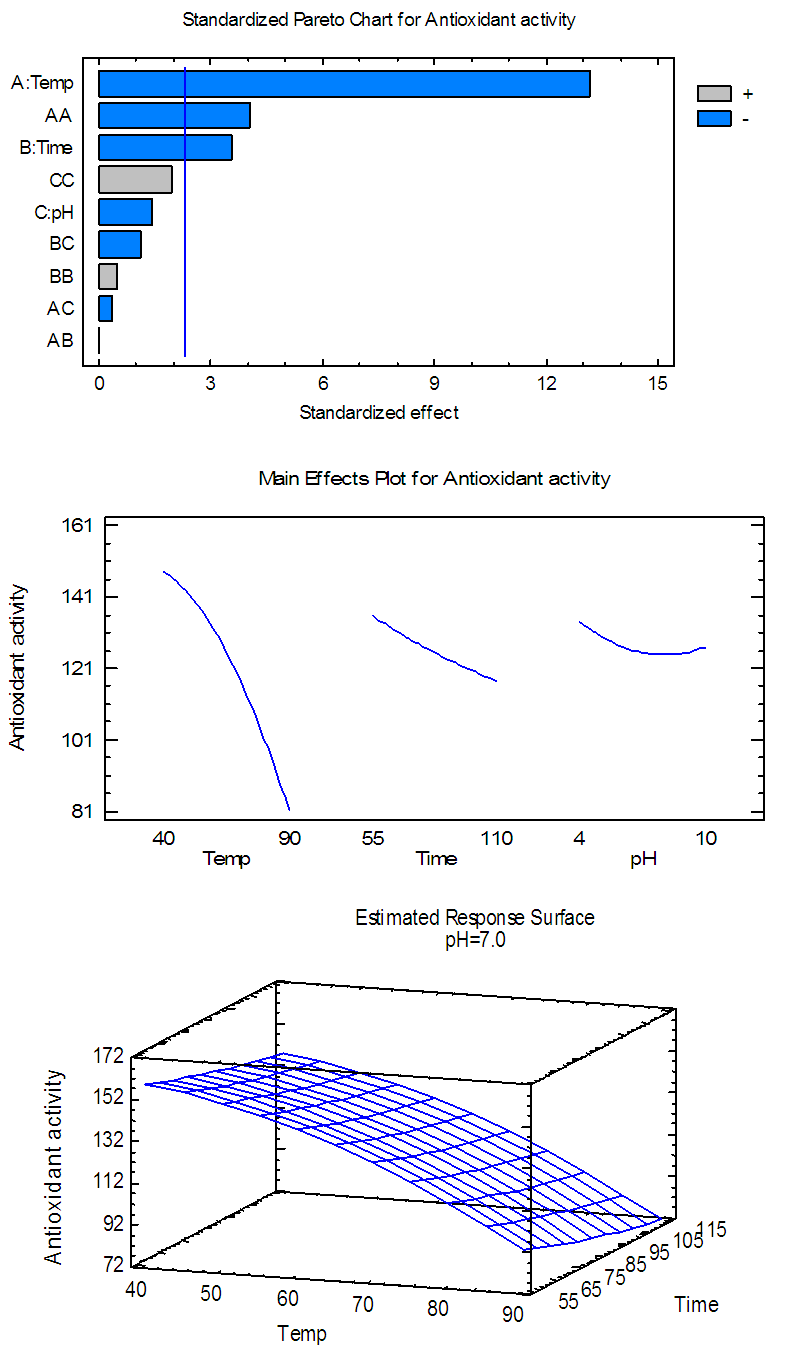 | Figure 4(c). Pareto chart, general trends, and response surface of the effects of temp., time and pH on AA for alcoholic Extract |
|
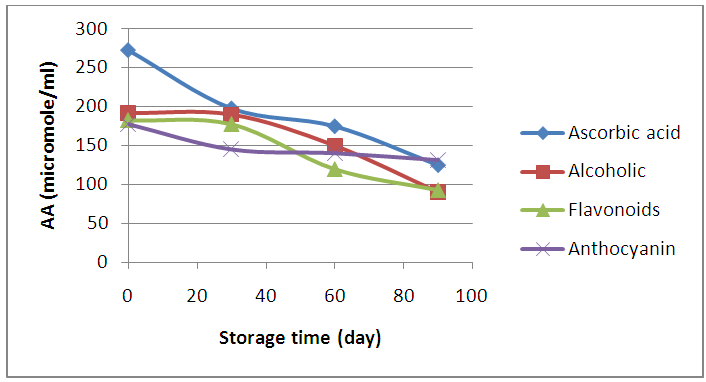 | Figure (5). Effect of storage time on the antioxidant capacity of alcoholic, flavonoids, and anthocyanin extracts of apple peels |
5. Conclusions
- Polyphonols are bioactive molecules which are very essential in our life as multifunctional health benefits ingredients. Extraction of these components from different natural resources including fruit waste contribute highly to the economics of industrial production and human needs. Peels of apples cultivated in Kurdistan region of Iraq (barware mountains) seemed to be rich natural sources for bioactive molecules which reflected the good antioxidant characteristics of the extracts. Response Surface Methodology seemed as an efficient tool to optimize the variables affecting the chemical activity of the extracted value-added compounds.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML