Karimi Abolfazl 1, Kargarrazi Maryam 2, Masoumi Masoumeh 2
1School of Chemical Engineering, University of Tehran, Tehran, Iran
2Department of Chemistry, Islamic Azadi University of Tehran, North Branch, Tehran, Iran
Correspondence to: Karimi Abolfazl , School of Chemical Engineering, University of Tehran, Tehran, Iran.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this research investigated nitrogen Adsorption on 13X Zeolite with VSA process. Acid-Wash is a chemical and alcohol wash is a physical modification. The samples were characterized by analysis, x-ray and IR spectroscopy before and after treatment. Adsorption was doing in 298K temperature and 2 bar pressure, amount of its calculated with integrate surface of breakthrough. In this research introduce new method that determined amount of adsorption with measured exhaust gases in regeneration stage. Capacity after acid wash and use vacuum increase from 396 mg/Kg in first cycle to 476 mg/Kg, then changing capacity under heating process was studied from 456 mg/Kg to 615 mg/Kg. Alcohol-Wash may decrease Zeolite 13X Nitrogen adsorption property.
Keywords:
Nitrogen, Adsorption, VSA, Acid & Alcohol Wash
Cite this paper: Karimi Abolfazl , Kargarrazi Maryam , Masoumi Masoumeh , Study of Nitrogen Adsorption in Modified 13X Zeolite with Acid & Alcohol Wash Method, Science and Technology, Vol. 6 No. 2, 2016, pp. 36-44. doi: 10.5923/j.scit.20160602.02.
1. Introduction
Vacuum swing adsorption (VSA) process is a developed operation to separate and purify Gasses, that is based on solid adsorption ability and selective keeping optional gases [1]. In order to air separating, cryogenic distillation classic method is the most important common way to generate oxygen. Study of this process as begun from decades 50, 60, but it has been commercial from decade 70 [2, 3]. Knowledge developed in production of Zeolite molecular sieve that selective, in adsorption Nitrogen with high efficiency, facilitates this condition to provide nitrogen production to get over 200 ton/day. VSA process have advantage for separation of air and production more than 15 ton/day oxygen in comparison to PSA, and is more Economic for less than 40 ton/day related to cryogenic Distillation method [4]. Zeolite structure contain SiO4 and AlO4 tetrahedrons joined by oxygen atoms. The isomorphic replacement of Al for Si raises a negative charge in the zeolite lattice. This net negative charge is balanced by the exchangeable cations (Na+). The acid treatment exchanges cation of zeolite with H+ and causes dealuminiumation through remove of Al from the frame work [5]. A Miller et al had studied in limestone based treatment of acid [6]. A Rivera et al had studied in Acid natural clinoptilolite for separation of n-paraffin's [7]. In this research VSA process for separation of nitrogen from air with zeolite 13X that has been modified with Acid-wash and Alcohol-wash method, Acid-wash method replaced on adsorbent cation with H+ and made changed inner and outlet surface activity, which is in agreement with our experimental results from this method can be used for increase efficiency and quality of zeolite adsorption in industrial scale. This idea composed used alcohol wash for eliminate of impurities from external & surface space of adsorbent that has been used an physical method.
2. Material & Apparatus
2.1. The Specification of Material
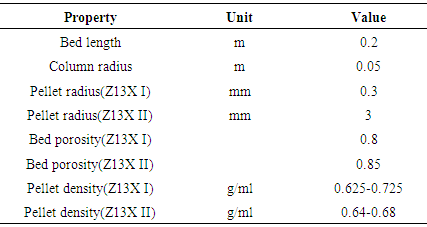
In these experiment have used two kind of Zeolite Which one of them was bought from ZEOCHEM company (Z13XI) to test in alcohol-wash experiment, and another one Z13XII was bought from AFRAZAND company use for acid wash experiment. Zeolite II size was larger than Zeolite I. Zeolite and its bed specifications are shown in table (1).Table 1. Bed and 13x zeolites characteristics
 |
| |
|
We used %37 chloride acid (HCL), Ethylic alcohol (C2H5OH, 96%), and re-distillated deionized water (0.001 mS/cm), glass bide (3mm), filter papers (90mm Φ).
2.2. Apparatus
There is a schematic diagram of apparatus in figure (1).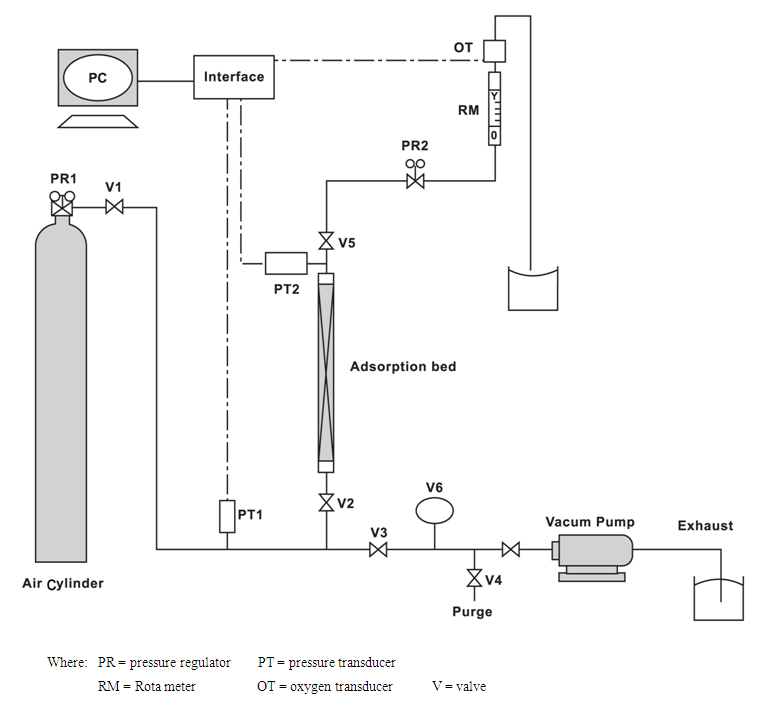 | Figure (1). A schematic diagram of an apparatus |
VSA process consists of 4 stages; pressurize, Adsorption, depressurize, and vacuum operation on every cycle. In order to do this process, we used Adsorption system as bench/scale including an Adsorption column with pipes (316SS, 1/4"), valves, Rota meter, oxygen sensor, vacuum pump (VALUE-VE115), hardware tool to transfer Data and special software to convert and monitor results. Two perforated plats with thin hole mash installed on both ends head of column to fix Adsorptions. Oxygen meter (LUTRAN company) with response time of 15-20 seconds placed in a hand-made case. Oxygen concentration will plot based on time scale in software environment. Sampling time or duration is adjustable. In this test, we consider 5 seconds for this experiment (it is a proper time, regarding to Adsorption rate). Moreover, since oxygen sensor should not be under Gas pressure, so we used a needle valve behind adsorbent column in front of sensor. As this as will pass from needle valve without existing any pressure above transmitter, and the oxygen concentration will measure. In vacuum operation, input & output (I/O) route will be closed and all nitrogen remains in bed, then evacuated from bypass path. The amount nitrogen absorbed in bed will remove. By using a pressure on Adsorption step, nitrogen molecules pierced in particles pores. In order to be sure of completely removing gasses molecules nitrogen, we put vacuum pump outlet pipe in a glass vessel water and whenever existing bubbles stops, it means that the bed is lacking of gas, that indicates the end of removing all gasses.
2.3. Preparation of Adsorbent
In order to activate Zeolite, we use two methods of acid & alcohol wash.
2.3.1. Acid Wash Method
We mix %5 dilute acid chloride solution with adsorbent completely at ratio of 1/5 and mix them for 5 minutes. In this process, H+ will be replaced instead of Z cation. Then we wash it with deionized water to remove all impurities for a several times. This function will continue, till the water under filter become clear and without chlorine ions, and its PH reaches to 6-7 level. Then after passing filter, dry it at 110c for 11hrs in oven.
2.3.2. Alcohol Wash Method
We mix Zeolite with alcohol at ratio of 3:2, then mix them, till solution become not any more turbid. After passing from filter, dry it at 110°C for 3 hr’s in oven.
3. Results & Discussion
3.1. x-ray Analyses
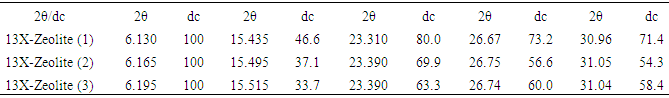
The results of x-ray diffraction (XRD) patterns of acidic and alcoholic as modified form and reference of 13X-Zeolites are shown in Table 2 because of the little move transfer (2θ) and intensity of preeminent peaks in zeolite samples are not significant; so, frame work distortion by acid and alcohol washing is giveback which was imported in this research. The difference between angles and peak intensities are related to modification by acid and alcohol washing. The hydronium ions in hydrochloric acid and ethylic alcohol activate the surface location on zeolite as adsorbent.Table 2. The values of 2θ, XRD patterns 13X-Zeolites (1-reF., 2-acid wash and 3-alcohol wash.)
 |
| |
|
As resulted the pattern, the intensity of main peaks were increased after modified by acid washing as well as cation exchange between (Na+ and H3O+) for N2 adsorptivity. In calcination process the porosity and channel of Zeolites are decreased.
3.2. IR Spectroscopy
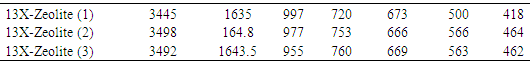
In addition to X-ray method, IR spectroscopy demonstrated important information about Zeolite structure, specially its structure location in X-ray diffraction.IR spectroscopy results are extensively used to characterize of zeolites as adsorbent systems before and after modification. In the case method, it is easier to carry out comparison the quantitative measurements in vibrations of the frameworks zeolites that give rise to typical bands. The main vibration give rise to bands in the fundamental stretch region at about as shown in table 3.Table 3. The wavenumber (cm-1) transmitted in 13X-Zeolites (1-ref., 2-acid wash., 3-alcohol wash.)
 |
| |
|
The bridging OH-group s (such as ≡ AL(OH) Si ≡ groups with Bronsted acidic character are increased in intensity and stretch regions. The nature of TO4 frame work in the (OH) Si configure ration is improved the N2 adsorption which obtained in modified zeolites after acid washing.N2 adsorption also enables zeolite OH- groups, especially acidic Bronsted sites to be characterized.The Oxygen concentration of outlet from apparatus is measured by sensor, then control and monitor in a software structure of LUTRON Company based on time, dynamically these data with eq1 converted to nitrogen concentration with function has been written in MATLAB program.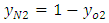 | (1) |
at first, Adsorption experiments will be done before treatment of 13X II as acid wash method, in order to evaluate the effect of this method. Breakthrough of nitrogen adsorption in two consequence cycle with operating condition of table 4 has been shown in figure 2.Table 4. Operating condition of Adsorption with Z13XII
 |
| |
|
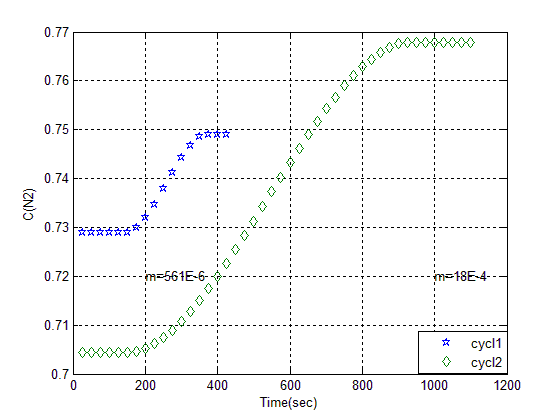 | Figure 2. Breakthrough of Z13XII in two consequence cycle |
The amount of nitrogen adsorption based upon mass unit of adsorbent from breakthrough with eq2 has been calculated.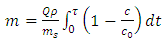 | (2) |
where:Q = volumetric flow rate of inlet feed (m3/s) ms = mass of adsorbent in the bed (kg)τ = constant time (s)co = nitrogen concentration in inlet feed (mol/m3)c = outlet nitrogen concentration from the apparatus (mol/m3)gas density ρ has been calculated with ideal gas law from eq3.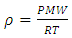 | (3) |
P (bar) is pressure of adsorption column and T (K) is operating temperature.The amount of nitrogen Adsorption is measured at adsorbent mass in cycle(1) = 516 mg/Kg, and in second one is 1800 mg/Kg, that shows Adsorption in 2 ndcycle is 3.2 times more than cycle one. Surface of adsorbent initially had coated with air molecules in neighborhood of them. In adsorption with increase of pressure to 2 bar nitrogen adsorbed to surface of adsorbent selectively. Finally in de sorption stage at the end of cycle all of the molecules in the surface and pore of adsorbent has been evacuated with use of vacuum so have a larger surface for gas to covered in the next cycle.
3.3. Acid Wash
This step will be done as explained in preparation of adsorbent section. Break through with same operating condition shown in figure (3).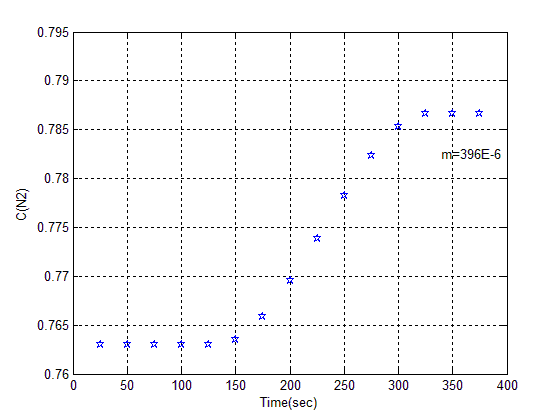 | Figure (3). Breakthrough of Z 13X II in acid wash method |
The Amount of adsorption from this curve has been calculated to 396 mg/Kg, different cationic state Specially acid form of its that made from acid wash process indicates has catalyze property, another experimental method is as follow for calculated exact amount of adsorption. Install rotameter after vacuum pump, immersed entrance pipe to glass of water. After adsorption stage closed v5 and open v2, measured amount of gas in the adsorption column which has shown in figure (4).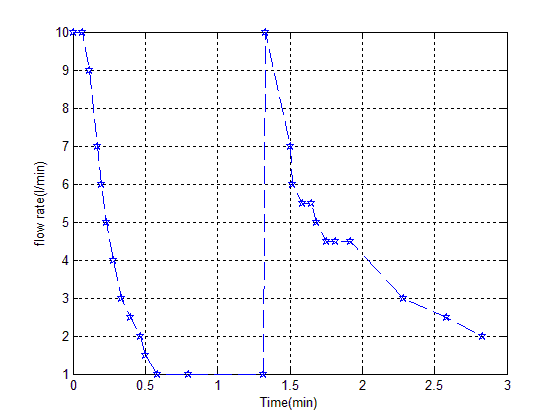 | Figure (4). Flow rate versus time in desorption stage |
In the first vacuum is turnoff and entrance gas with column pressure in adsorption stage. Amount of gas calculate with eq(4).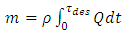 | (4) |
In this process, Z cation (Na+) is replaced by H+, Moreover, by increasing porosity and special surface, we observe Adsorption property as a chemical trait of Z, and according to this, its concentration will change. Main structure or topology of zeolite determine pore size, porosity, cation change from view of location of its in structure, population density, load and amount of its could be modified behavior molecular sieve property, selectivity and separation factor [8].Cations are under effect of electrical field, related to location in lattice with absorbed material molecules. On following regenerated of the bed with used of vacuum amount of nitrogen in further adsorption has been got 476 mg/Kg that indicate increase of 20%. This is demonstrate evacuate nitrogen from the macro pore. On following in amount of nitrogen in consequent cycle is 465 mg/Kg. this indicate decrease of capacity of adsorption and incomplete of desorption. So remove zeolite from column, and then put it on oven for 90 min on 120°C. In this way N2 Adsorption will reach to 615 mg/Kg, that shows %24 increasing on Adsorption. The surface of a adsorbent may be specified by its pore size, Small pores have higher shares. In fact, their shares is so more that could be ignore of external surface to calculate surface of adsorbent, through increasing temperature, might grow crystal in various cell units, and pore volume also changes. Large typical surface indicates that a high activity is arise but by comparison, we found that Z adsorption activity depends on not only volume of their surfaces, but also depends on pores surfaces, which are available and in access combined material in Adsorption process [8], but in high temperature pores will be destroyed. Nitrogen inside Z crystal is tailed between group of cation bonds or oxygen molecules. The amount crystal that has occupied by nitrogen, might be %50 of total crystal volume [9]. After heating process, vacant space will appear that is available for other molecules. Breakthrough of adsorption in three sequential cycle is shown in figure (6). The amount of nitrogen adsorption is (365, 219 and 199) mg/Kg that indicates they were reached on a fix level.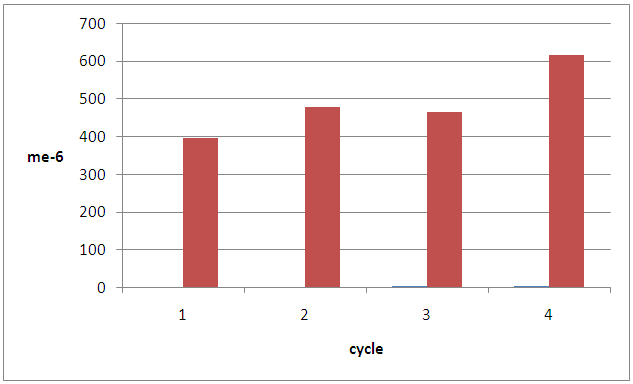 | Figure (5). Compare amount of adsorption in consequence cycle |
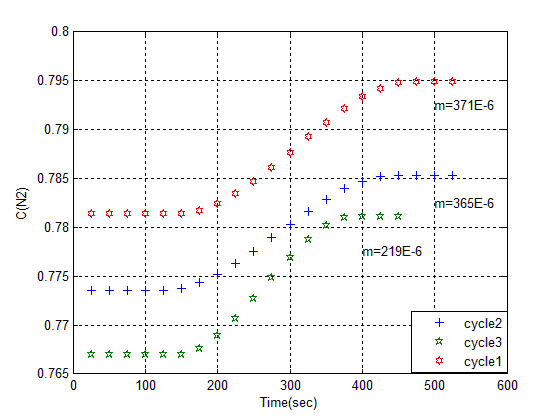 | Figure (6). Breakthrough of 3 sequential cycle with acid wash Z |
3.4. Alcohol Wash
Since Z (13X I) had not nitrogen Adsorption, in order to revive it, we should put it in oven at 110°C for 3 hrs. (see figure 7).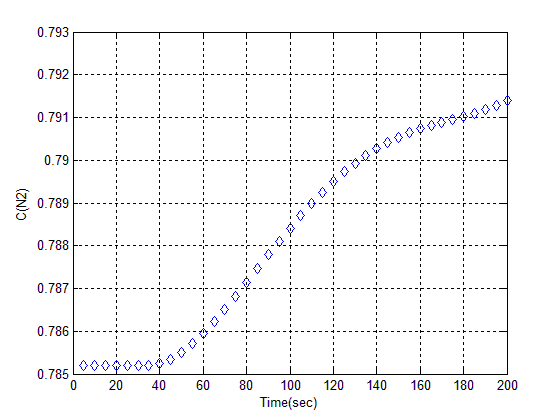 | Figure (7). Curve of Z (13 X I) |
In order to study and specify whether Ethylic alcohol is just a nitrogen adsorbent or not, we used 400gr glass bide with 100gr smeary Ethylic alcohol on adsorbent column. This test is done as previous condition, through passing air, we found that Ethylic alcohol does have low intention to Adsorption of N, alcohol wash method is done with 150% ethylic alcohol on Z13XI that describe on preparation of adsorbent section. Adsorption experiment on this bed has been done in two consequence cycle its breakthrough has shown in figure (8). In this condition purity changing is low.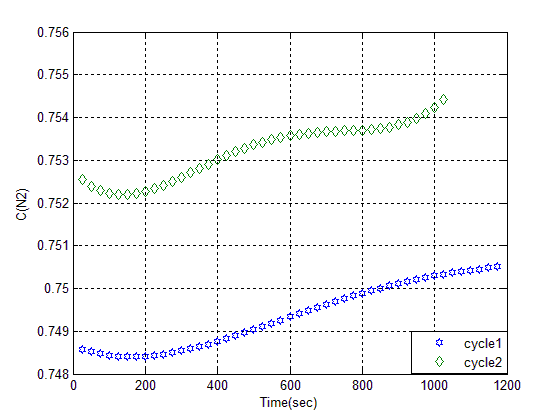 | Figure (8). Breakthrough curves of alcohol wash bed |
On the following, in order to study better, we washed bed with less alcohol. In order to ready an reuse adsorbent, put Z in oven at 120c for 24hrs. The adsorption on two consequence cycle is shown in figure (9).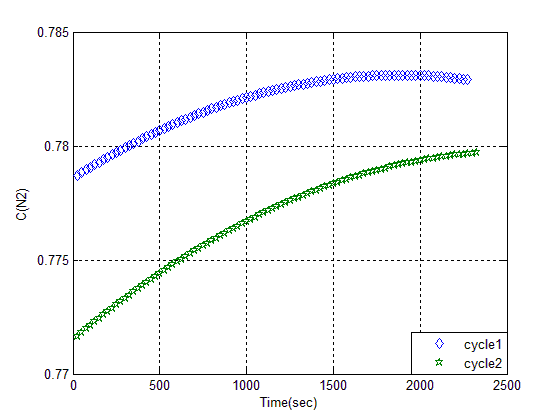 | Figure (9). Breakthrough of regenerated heat adsorbent Z (13X I) after alcohol wash |
These results proved that related Z in this condition is a N adsorbent. By using heat, its property will improve in the first experiment, Z was washed with %10 alcohol, and mixed 10 min, so that, solution color did not change any more. During mixing, a large amount of solution attracted to inner pores of Z. This action is exothermic, thus we filtered it and put it in oven for 2hrs at 120°C. Its breakthrough are respectively %10 and %20. (see figure 10).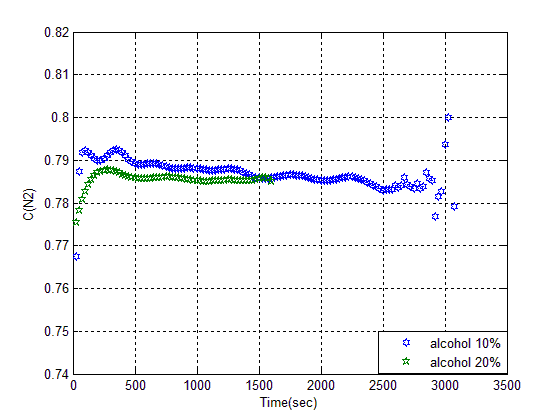 | Figure (10). Breakthrough curves of alcohol wash with 10, 20% adsorbent |
We found that decrease N adsorption, therefore, we use more percent alcohol, but did not happened any changes. This result show that with alcohol wash method hydroxide (OH) coated adsorption site on inner surface of particle and causes reduce nitrogen adsorption property of (Z13 X I).
4. Conclusions
One of the ways of modify Z structure, is acid wash method that acidic state of Z ready through direct ion exchange with acid. The experimental results show that size, form of adsorbent molecule, and its structure would change in acid wash method, that has impact on capacity of adsorption process. The findings show, when adsorbent is exposed under heat, nitrogen exists from adsorbent micro pores .From the view of thermodynamic nitrogen capturing inside micro pores, shows weak value bond, that its enthalpy increases by accretion heat, thus, this bond ill break and separate, indicates through hydrodynamic changes, we will not be able to remove all absorbed nitrogen, but by doing thermodynamic method, ill bonds will become weak and adsorbent would be lack of N. The results show that, we finally may access to fixed adsorb value.
References
| [1] | Ralph T. Yang, “Adsorbents, Fundamentals and Applications”, 2003 by John Wiley & Sons, Inc. |
| [2] | Yuwen, Z., Yuyuan, W., Jianying, G., Jilin, Z. The experimental study on the performance of a small-scale oxygen concentration by PSA, J. Separation and Purification Technology, 2005; 42: 123–127. |
| [3] | Zhang, J., Xiao, P., Li, G., Webley, P. A. Effect of Flue Gas Impurities on CO2 Capture performance from flue gas at coal-fired power stations by vacuum swing adsorption, J. Energy Procedia 1, 2009: 1115–1122. |
| [4] | JEE, J.G., JUNG, J. H., LEE, J.W., SUH, S.H., LEE, Ch.H. Comparison of vacuum swing adsorption process for air separation using zeolite 10x and 13x, J. Revue Roumaine de Chimie, 2006; 51(11): 1095–1108. |
| [5] | Burcu Erdogan Alver, “A comparative adsorption study of C2H4 and SO2 on clinoptilolite-richtuff: Effect of acid treatment”. journal of Hazardous Materials 262(2013) 627-633. |
| [6] | Andrew Miller., Thomas Wildemanb, Linda Figueroa “Zinc and nickel removal in limestone based treatment of acid mine drainage: The relative role of adsorption and co-precipitation”. Applied Geochemistry 37 (2013) 57–63. |
| [7] | Aramis Riveraa, Tania Faríasa, Louis Charles de Ménorvalb, Giselle Autié-Castro, “Acid natural clinoptilolite: Structural properties against adsorption/separation of n-paraffins”. Journal of Colloid and Interface Science 360 (2011) 220–226. |
| [8] | Othemer, K., et al. (2007), “Encyclopedia of chemical & technology”, vol.16:811-853 2-Z. BUDNER, J.DULA, W. PODSTAWA, Trans ICHEME, VOL 77, PartA, july1999. 3-Y.Y.LI, S.P. Perera and B.D. CRitenden, Trans I Cheme, vol 76, part A, November 1998. |
| [9] | Friedel, G., Bull.Soc.Franc. Min., Paris, 19, 24 (1896). |
| [10] | Inc.pub. Madison; “Methodsofsoil analysis”, part1-2, (1969), American Soc. of Agronomy, Wisconsim, U.S.A. (chapter: 21, 29, 57). |
| [11] | R. Jenkins and J.L. de Vries; “An Introduction to x-ray powder diffractometry”, Philips company. |
| [12] | Nato ASIseries: Martinus Nijhoff publishers (1984). “Zeolites: science and technology”. No.80. |
| [13] | Slavica Lazarevića, Ivona Janković-Častvana, Dušan Jovanović. “Adsorption of Pb2+, Cd2+ and Sr2+ ions ntonatural and acid-activated sepiolites”. Applied Clay Science 37 (2007) 47–57. |
| [14] | Soydoa Vinitnantharat, Sriwilai Kositchaiyong, Siriluk Chiarakorn. “Removal of fluoride in aqueous solution by adsorption on acid activated water treatment sludge”. Applied Surface Science 256 (2010) 5458–5462. |
| [15] | Cristina Volzone, Jose Ortiga. “SO2 gas adsorption by modified kaolin clays: Influence of previous heating and time acid treatments”. Journal of Environmental Management 92 (2011) 2590e2595. |



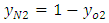

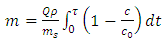
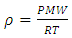


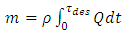






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


