-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2015; 5(1): 15-19
doi:10.5923/j.scit.20150501.03
The Effect of Water Sorbent on Lipase-Catalysed Esterification of Fatty Acid
Isaac K. Frimpong , Robert D. Nagre , Lawrence Nti
Department of Chemical Engineering, Kumasi Polytechnic, Kumasi, Ghana
Correspondence to: Isaac K. Frimpong , Department of Chemical Engineering, Kumasi Polytechnic, Kumasi, Ghana.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
High conversions in lipase-catalysed production of fatty acid esters from fatty acids and alcohols require efficient removal of water preferentially. A lipase-catalysed esterification of oleic acid and dodecanol using adsorbent (zeolite) and absorbent (superabsorber) was investigated. The zeolite and the superabsorber were used to shift the equilibrium toward fatty acid ester production by preferential elimination of the water by- product during the esterification reaction. Improved products of fatty acid esters with lower acid values were obtained for both the zeolite and superabsorber systems. The superabsorber exhibited better water removal property in shifting the equilibrium toward the desired product compared to the zeolite.
Keywords: Esterification, Fatty acid ester, Lipase, Zeolite, Superabsorber
Cite this paper: Isaac K. Frimpong , Robert D. Nagre , Lawrence Nti , The Effect of Water Sorbent on Lipase-Catalysed Esterification of Fatty Acid, Science and Technology, Vol. 5 No. 1, 2015, pp. 15-19. doi: 10.5923/j.scit.20150501.03.
Article Outline
1. Introduction
- The products from chemical and process industries have been an indispensable commodity in modern day life. These industries are, however grappling with the need to improve upon these products as well as handling the industrial wastes that are generated. Industries are therefore highly demanded to develop products and processes that are sustainable. Raw materials and advanced methods must be carefully selected to achieve high conversion rates and efficient use of energy. Therefore, the existing and future design processes must exceed those achieved by using the toolbox of conventional process units to overcome these challenges [1]. This necessitated the introduction of process intensification [2-4] in recent years, which Lutze et al., [4] described as a tool for the targeted improvement of phenomena that occur at different scales to achieve a targeted benefit. The ultimate aim at the reaction stage is to attain the highest production efficiency and yield of the desired product in a safe operation as well as the highest recovery and purity of products at the separation steps in order to maximize profit. [5].Esters are one of the most useful classes of organic compounds in the chemical and process industries. Esters, are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst.
 | (1) |
2. Experimental Methods
2.1. Chemicals
- Oleic acid was bought from Merck KGaA (Darmstadt, Germany, 65-88%). For the esterification, dodecanol (Merck Schuchardt OHG, Hohenbrunn, Germany, 98%) and biocatalyst (lipase), Novozyme 435 (Novo, Denmark) were used. The zeolite and the superabsorber were obtained from Stockhausen GmbH, (Krefeld, Germany). Differential thermogravimetry (DTG) and differential thermal analysis (DTA) were carried on the zeolite. The analysis (Figure 2) shows that, it is able to withstand a maximum temperature of about 300℃. The zeolite was activated in a drying oven overnight at 250℃ prior to use.
2.2. Procedure
- The experimental set-up used for the esterification reaction (3) is schematically shown in Figure 1. The 250- ml three-neck round bottom flask was placed in a thermostatic water bath and a thermocouple was used to regulate its temperature. The esterification reactions were carried out in a fixed-bed reactor. The reactor was filled with the bio-catalyst together with glass beads in a ratio of enzyme to glass beads (vo/vo) of about, 1: 3. The reaction temperature of 50℃ was maintained by circulating heated water. A filter tube was filled with zeolite (and superabsorber in a separate experiment) and inserted into the neck of the flask through which the reaction mixture returns to the flask. The zeolite and the superabsorber were used primarily to adsorb and absorb water respectively. An equimolar ratio of oleic acid and dodecanol was used in the experimental. Oleic acid was put in the flask and heated up to the reaction temperature. Dodecanol was heated separately to the reaction temperature and then added to the flask. The reaction mixture was simultaneously pumped continuously through the reactor unit at the rate of 1.43ml/s. The total volume of the reaction mixture was 350- ml. Samples were withdrawn periodically to determine the acid values based on AOCS Cd 3d-63 procedure [15]. The acid value was calculated using the formula in (1). The esterification of oleic acid with dodecanol, without removing water was also carried out.
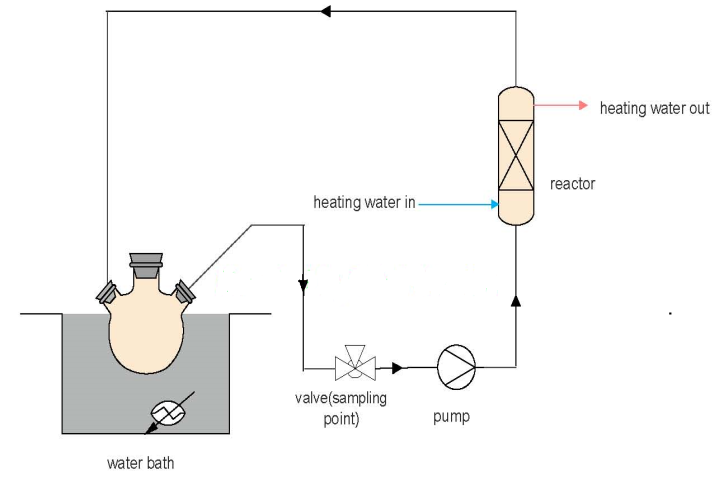 | Figure 1. Process flow diagram of liapse-catalysed esterification of and oleic acid dodecanol |
 | Figure 2. DTA and DTG analysis of zeolite |
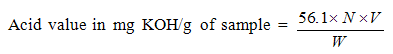 | (2) |
 V = volume(ml) of KOH used
V = volume(ml) of KOH used W = weight of sample in grams(g)
W = weight of sample in grams(g)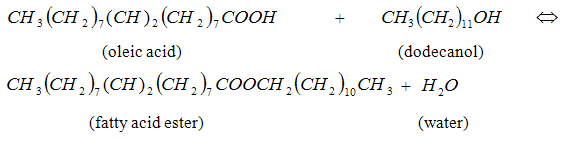 | (3) |
3. Results and Discussion
- The acid values obtained in the reactions are presented in Figure 3. The results indicate that both the zeolite (adsorbent) and the superabsorber (absorbent) were effective in preferentially removing water during the esterification to yield fatty acid esters with lower acid value. A uniform reference initial acid value for all the esterification reactions was calculated to be 122.4 mgKOH/g. The esterification of oleic acid and dodecanol without removing the water by-product resulted in a higher acid value of the ester 15.70 after 210min of reaction. The introduction of the adsorbent and the absorbent into the reaction system significantly reduced the acid value from 15.70 mgKOH/g to 2.24 mgKOH/g and 1.68 mgKOH/g respectively. However, the results show that the superabsorber yielded a substantial lower acid value than zeolite at the same reaction time (210min) indicating the superabsorber was a better water remover compared to the zeolite. These results were obtained at a reaction temperature of 50℃ and an equimolar ratio of fatty acid to dodecanol.
 | Figure 3. Effect of time on Acid value for esterification of oleic acid and dodecanol for ( ) normal reaction without water removal and water removal ( ) normal reaction without water removal and water removal ( ) with zeolite and ( ) with zeolite and ( ) with superabsorber ) with superabsorber |
4. Conclusions
- A lipase-catalysed esterification of oleic acid with dodecanol was successfully carried out with equimolar amount of the acid and the alcohol at a temperature of 50℃. Zeolite adsorbent and absorbent were used to selectively remove water during the reaction to displace the equilibrium towards the products. Both the adsorbent and the absorbent system yielded substantially low acid value of 2.24 and 1.68 respectively after 210min of reaction time. However, the superabsorber exhibited superior water removal property compared to the zeolite. The superabsorber could be regenerated for reuse whilst the zeolite could not.
ACKNOWLEDGEMENTS
- The Authors wish to thank the management of Kumasi Polytechnic for the massive support for this work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML