-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2012; 2(6): 168-171
doi: 10.5923/j.scit.20120206.05
Design, Fabrication and in Vitro Evaluation of Metformin HCl Matrix Tablets for Treatment of Diabetes Mellitus
Shyamoshree Basu Ghosh , Sumit Mukherjee
NSHM College of Pharmaceutical Technology, NSHM Knowledge Campus, Kolkata, West Bengal University of Technology, 700053, Kolkata
Correspondence to: Shyamoshree Basu Ghosh , NSHM College of Pharmaceutical Technology, NSHM Knowledge Campus, Kolkata, West Bengal University of Technology, 700053, Kolkata.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Diabetes mellitus is a growing problem in today’s world. The aim of the present study was to design and develop matrix tablets of metformin HCl (MTH) with hydroxy propyl methyl cellulose K15M (HPMCK15M) and ethyl cellulose (EC) in different ratios. The prepared tablets were evaluated in terms of hardness, friability, drug content and in vitro drug release profiles. It was observed that formulation with HPMC and EC in a ratio of 2:1 gave reproducible results in all aspects and showed sustained drug release profile for 12h.
Keywords: Diabetes Mellitus, Matrix Tablet, Metformin Hcl
Cite this paper: Shyamoshree Basu Ghosh , Sumit Mukherjee , "Design, Fabrication and in Vitro Evaluation of Metformin HCl Matrix Tablets for Treatment of Diabetes Mellitus", Science and Technology, Vol. 2 No. 6, 2012, pp. 168-171. doi: 10.5923/j.scit.20120206.05.
Article Outline
1. Introduction
- Diabetes mellitus is rapidly becoming a serious threat to human health. According to World Health Organization estimates, number of people with diabetes worldwide, in 2000, was 171 million, which is likely to increase at least 366 million by 2030[1]. This increase will be most noticeable in developing countries, where the number of people with diabetes is expected to increase from 84 million to 228 million. According to the WHO, Southeast Asia and the Western Pacific region are at the forefront of the current diabetes epidemic, with India and China facing the greatest challenges. In these countries, the incidence and prevalence of type 2 diabetes among children are also increasing at an alarming rate, with potentially devastating consequences[2]. Metformin HCl (MTH) is an anti-diabetic drug which is prescribed for the treatment of non-insulin dependent diabetes mellitus[3]. In addition, it has a favourable action on cardiovascular risk factors, which are often present in these individuals. It helps in maintaining diet-induced weight loss and lowers fasting plasma insulin concentrations, total and low density lipoprotein-cholesterol, and free fatty acids. These effects make MTH a first-line agent for the prevention of type 2 diabetes[4]. The average MTH elimination half‐life in plasma is 6.2h and its peak plasma concentration (Cmax) is achieved within 4 to 8 hours with extended‐release formulations. In spite of its favourable clinical response and lack of significant drawbacks, chronic therapy with MTH suffers from certain specific problems of which, the most prominent being the high dose (1.5-2.0 g/day), low bioavailability (60%), high incidence of gastrointestinal (GI) tract side effects (30% cases) and decreased absorption of the drug with food that delays time to reach maximum drug concentration in blood (tmax) by half-an-hour[5, 6]. Thus there exists a potential for MTH to be used in the sustained release formulation tablets. The primary benefit of sustained release preparations of MTH compared to an immediate release formulation is that a more uniform maintenance of blood plasma active concentration can be achieved, potentially avoiding undesirable peaks and troughs associated with multiple immediate release preparation applications.Hydroxy propyl methyl cellulose (HPMC) is the most commonly and successfully used hydrophilic polymer for the preparation of oral controlled drug delivery systems[7]. Upon contact with the gastrointestinal fluid, HPMC swells, gels, and finally dissolves slowly[8]. The gel becomes a viscous layer acting as a protective barrier to both the influx of water and the efflux of the drug insolation. Volume proportion of the polymer in the formulation increases, the gel formed is more likely to diminish the diffusion of the drug and delay the erosion of the matrix. The dissolution can be either disentanglement or diffusion controlled depending on the molecular weight and thickness of the coating layer. The rates of polymer swelling, dissolution and drug release are found to increase with both increasing levels of drug loading or with use of lower viscosity grades of HPMC. Additionally, use of hydrophobic polymers significantly slows the drug release. So in the present investigation, an attempt has been made to formulate the extended – release matrix tablets of MTH using hydrophilic matrix material HPMC K15 M in combination with hydrophobic ethyl cellulose.The present study focuses at the development and optimization of an extended release formulation (matrix tablet) of MTH HCl for the treatment of diabetes mellitus.
2. Materials and Methods
- Metformin HCl was obtained as a gift sample from Ranbaxy Laboratories Limited, India. Hydroxy propyl methyl cellulose K15M was obtained as a gift sample from Colorcon Asia Pvt Ltd. Ethyl cellulose, Micro crystalline cellulose, Magnesium stearate and talc were purchased from S.D. Fine Chemicals.
2.1. Preparation of the Matrix Tablet
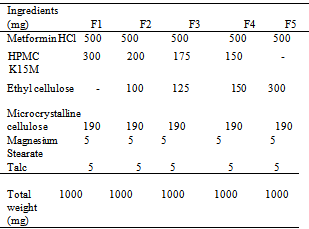
- Matrix tablets were prepared by non-aqueous wet granulation method. The composition of various formulations is given in Table 1. MTH HCL, HPMC K15M, EC, MCC were weighed accurately and individually passed through a 60 mesh and the mixture was passed through sieve mesh No. 40. The powdered ingredients were compressed into tablets on a 10-station mini rotary tableting machine with 12-mm round punches at a constant rotational speed of 72 rpm.
|
2.2. Physicochemical Evaluation of Metformin HCl Matrix Tablets
2.2.1. Hardness Test
- Hardness of 6 tablets was determined using a hardness tester (EH- 01, Digital hardness tester, Electrolab, India). Hardness values were reported in kilograms (kg/cm2)[8]. Mean and SD were calculated.
2.2.2. Friability Test
- 20 tablets were weighed and placed in a friabilator (EF-2 Friabilator, Electrolab, India) and subjected to 100 rotations for 4 min. The tablets were then dedusted and reweighed. The friability was calculated as the percentage weight loss[9].
2.2.3. Weight variation
- 20 tablets of each formulation were weighed using an electronic balance. Weight values were reported in milligrams. Mean and SD were calculated[10].
2.2.4. Drug content
- To determine drug content, 6 tablets of each formulation were weighed and crushed to powder with mortar and pestle. 300 mg of powder was taken and dissolved with appropriate amount of methanol with the aid of sonicator. After which the solution was filtered through filter paper. The total amount of the drug in the tablet was analysed after appropriate dilution of test solution by using UV spectrophotometer (UV-VIS Spectrophotometer, Shimadzu, Japan) at 233 nm against the reference solution with suitable dilution.
2.2.5. In Vitro Drug Release Study
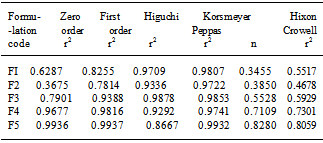
- In vitro drug release study of each formulation was determined using the USP Type II Dissolution apparatus (Labindia Instruments, India) where 900 ml of phosphate buffer saline (pH 6.8) were used as dissolution media and maintained at 37±0.50℃ at 50 rpm. The release rate experiments were conducted in 0.1 N HCl for 2 h and thereafter in phosphate buffer saline of pH 7.4 for 12 h. Aliquots of 1 ml were withdrawn at fixed intervals and replaced with fresh media. Then samples were analysed in the same spectrophotometer at 233 nm, keeping the dissolution media as reference sample. In order to determine the release model which best describes the pattern of drug release, the in vitro release data were fitted to zero order, first order and diffusion controlled release mechanisms according to the simplified Higuchi model[11]. The selection of a preferred mechanism was based on the correlation coefficient (r) for the parameters studied, where the highest correlation coefficient is preferred for the selection of mechanism of release. Data analysis was carried out using the following Korsmeyer and Peppas[12]
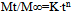 | (1) |
3.Results and discussion
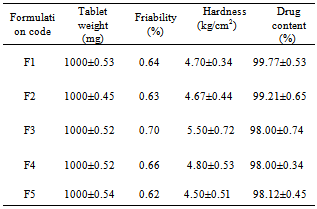
- Tablets of different formulations were subjected to various evaluation tests, such as weight variation, drug content, hardness, friability, and in vitro dissolution study. The average weight variation of 20 tablets of each formulation was less than ± 5%. Drug content was found to be uniform among different batches of the tablets and ranged from 98.00±0.34 to 99.77±0.53 %. The hardness and percentage friability of the tablets of all batches ranged from 4.50 ±0.51 to 5.50 ± 0.72 kg/cm2 and 0.62% to 0.70%, respectively (Table 2). Tablets of all formulations showed uniform thickness. In a weight variation test, the pharmacopoeial limit for the percentage deviation for tablets of more than 250 mg is ± 5 %. The average percentage deviation of all tablet formulations was found to be within the above limit, and hence all formulations passed the test for uniformity of weight as per official requirements[10]. Conventionally, the compressed tablets that lose less than 1% of their weight are generally considered acceptable. In the present study, the percentage friability for all the formulations was below 1%, indicating that the friability is within the prescribed limits. All the tablet formulations showed acceptable pharmacopoeial properties and complied with the prescribed specifications for weight variation, drug content, hardness, and friability.Table 2 contains a summary of the above parameters measured for five different formulations F1–F5 (corresponding compositions – see Table 1)
|
|
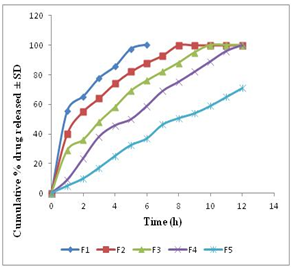 | Figure 1. In vitro meformin HCl release profile from matrix tablets. Data show mean ± SD (n=6) |
4.Conclusions
- From the above study it can be inferred that matrix tablet prepared with HPMC and EC in a ratio of 2:1 showed desirable results in terms of hardness, friability, drug content and in vitro drug release profiles.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML