-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2012; 2(5): 109-113
doi: 10.5923/j.scit.20120205.01
Study on Growth Habit, SHG and Thermal Properties of Urea Phosphate Doped KDP Crystals
G. G. Muley
Department of Physics, Sant Gadge Baba Amravati University, Amravati, 444602, (MS) India
Correspondence to: G. G. Muley , Department of Physics, Sant Gadge Baba Amravati University, Amravati, 444602, (MS) India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Pure and doped with Urea Phosphate single crystals of Potassium Dihydrogen Phosphate (KDP) were grown by the low temperature solution growth method, adopting the technique of slow evaporation of the solvent for nonlinear optical applications. The change in the growth habit of the doped KDP crystals has been reported. The study confirms the decrease in the growth rate along c-axis with increasing the dopant concentration. The doping of the Urea Phosphate in the grown crystal has been confirmed qualitatively by the FT-IR spectroscopy. Form the UV-Visible-NIR transmission study; it was observed that the transparency of the crystal increases with dopant concentration. Thermo Gravimetric Analysis shows that the thermal stability of the crystal decreases with dopant concentration. The crystal structure of doped crystal was determined from Powder X-Ray diffraction studies. The improvement in Second Harmonic Generation efficiency of doped crystal has also been reported.
Keywords: NLO crystal, Growth habit, Second harmonic generation, XRD study
Article Outline
1. Introduction
- Potassium Dihydrogen Phosphate (KDP) crystal is most widely used and thoroughly studied nonlinear optical (NLO) crystal. The attempts have been made to modify the properties favorable for NLO applications and growth rate of the KDP crystal by either changing the growth conditions or by adding different impurities[1-6]. The Ethylenediamine tetra acetic acid (EDTA) inhibits growth of KDP and acts as scattering centers in grown crystals[7] while on the other side it prevents number of nucleation’s, increasing metastable zone width, which helps in growing big size good quality crystal[8]. Azo-organic dye Amaranth gets absorbed and colors the pyramidal section (1 0 1) of the crystals and Sunset yellow FCF modifies the crystal habit and color of KDP. It decreases size of prismatic section of the crystal[9]. The increase in mean growth rate along the[001] direction has been reported with an increase in the concentration of trivalent impurity Cr(III) in the KDP solution while the growth rate along[100] direction is not altered[10]. A similar effect of Fe(III) and Cr(III) on the growth rate of KDP crystals has been reported by Owczarek and Sangwal[11]. Amino acids have very large optical nonlinearity, assigned to the chiral structure, used by many workers to improve the NLO, optical, thermal and mechanical properties of KDP using as a dopant[12,13]. Urea has been reported as a NLO material. Although it’s optical and mechanical properties are comparable to those of KDP, but its crystallization and handling is rather difficult as it is highly hygroscopic. Different derivatives of urea have been studied for NLO applications. Some of them found useful for NLO applications[6,14-18]. The Urea and Urea derivates Thiourea, N’N dimethyl Urea have been tried as a dopent in KDP[19-22].As KDP is being widely used in NLO applications and demands to modify its properties, the research work on the modifications of the optical, thermal, mechanical and NLO properties and the growth rate of the KDP crystal by using different dopants is still going on[23-25]. The urea and urea derivatives have high NLO efficiency and can be used as dopant to modify the properties of KDP crystal. In the present work, an attempt has been made to catch the properties of Urea derivative; Urea Phosphate and add it with KDP to investigate the effects. The growth of Urea Phosphate doped KDP crystals and their characterizations have been presented. Improvement in the SHG efficiency has been reported. Modification in the growth habit has also been observed.
2. Experimental
2.1. Bulk Crystal Growth
- The solubility’s of the pure (KDP) and 2mol%, 4mol% and 6mol% Urea Phosphate doped KDP (KU1, KU2 and KU3 respectively) crystals were measured gravimetrically in double distilled water in the temperature range 30-60℃ (Figure 1). The materials have positive solubility gradient with temperature in water. The double distilled water was used as a solvent throughout the experiment.Good quality crystals of pure and doped KDP were grown within 3-4 weeks. In doped crystals, growth rate along c-axis is found to be decreased. The pH of the solutions was ranged from 4.5 to 4.7. The photographs of some of the grown crystals are shown in Figure 2.
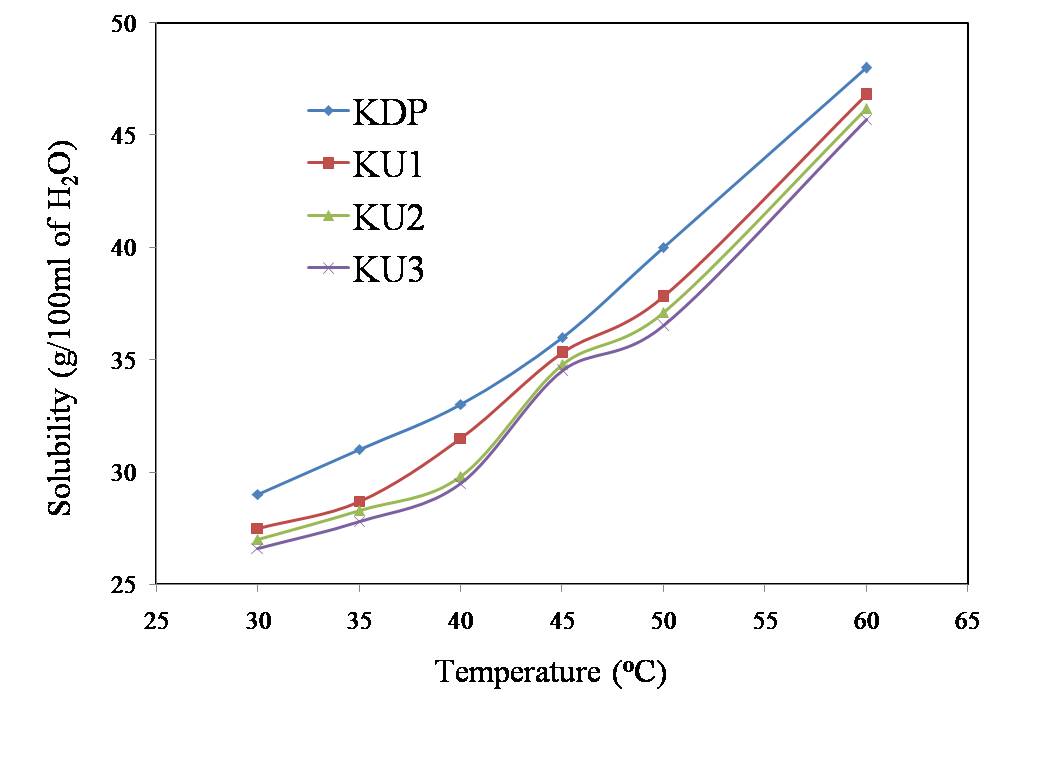 | Figure 1. Solubility curves |
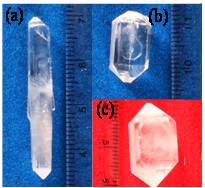 | Figure 2. Photographs of (a) KDP, (b) KU1, (c) KU3 crystals |
2.2. Characterization
- The FT-IR study was carried on the pure and doped KDP crystals to confirm qualitatively, the doping of the dopants in the crystals. Transparency of the crystals in the wavelength range 190-1100nm was studied by using UV-1700 SHIMADZU Spectrophotometer. Powder XRD patterns were recorded on XPERT Pro Diffractometer and analyzed by Powder X software. The results have been presented. SHG efficiency was measured by employing Kurtz and Perry method. The SEM images of the crystals on the pyramidal section (1 0 1) were taken.
3. Results and Discussion
3.1. Growth Habit
- The morphology of KDP crystals has tetragonal prismatic (100) and (010) faces and two tetragonal pyramids with the faces (101) and (011). The chemical bonds formed in KDP crystal between the growth units are the ones between K+ cations and H2PO4– anions, as well as the hydrogen bonds of the adjacent H2PO4– groups. The prismatic faces of KDP grow by stacking positive K+ and negative H2PO4– ions alternately leaving the face {100} charged neutrally and adsorbs the metal cations and organic molecules, which can form hydrogen bonds. The pyramidal growth sectors {101} are stacked in the mode of two layers of cations and two layers of anions alternately. Each layer consists completely of either cations or anions. The potassium ions join into the lattice site rapidly as the negatively charged double layer (H2PO4–) is formed, because of its small volume, light weight and no preferred orientation required as compared with H2PO4–. Thus, the pyramidal face is always positively charged throughout the growth process. The anions get adsorbed on the positively charged (101) planes and continues growth of the crystal in the direction[26]. It has evidences that the metallic cations and dyes influence the growth of prismatic (100) section and pyramidal (101) section of KDP crystals[27-31].In the present study, the growth rate of doped crystals along c-axis is found to be decreased. In the Urea Phosphate doped KDP crystals, the decrease in the growth rate along c-axis, is possibly because of accommodations of the bigger size anion CO(NH2)2.H2PO4- on the pyramidal section at the place of relatively smaller size anion H2PO32-, moreover two groups; CO(NH2)2 and H2PO4- are bounded by hydrogen bonding, have less affinity to other anions CO(NH2)2H2PO4- as compared to the H2PO4- groups in pure KDP, as a result, growth rate decreases along[001] direction. In the SEM image (Figure 3) of the pure KDP, the surface appears rough as compared to the doped crystal, showing more sites for the attachments of the ions; cations and anions.
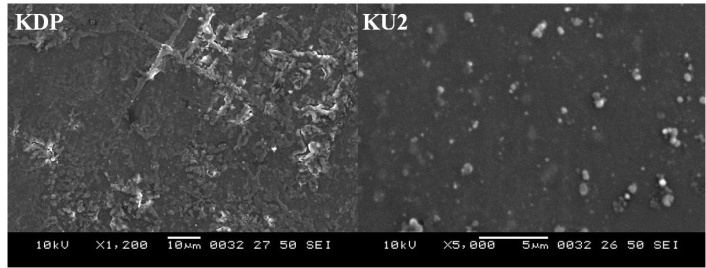 | Figure 3. SEM images of the (101) plane |
3.2. Fourier Transform Infrared (FT-IR) Study
- The FT-IR spectra of pure and doped KDP crystals (Figure 4) were recorded on Perkin Elmer FT-IR Spectrophotometer within the wavenumber range 600 to 4000 cm-1.In the FTIR spectrum of pure KDP crystal, the observed absorption peak at 3734.1cm-1 corresponds to the P-OH stretching, 2365.12 cm-1 to O-H and P-OH stretching, 1750.24 cm-1 to the P-O-H bending, 1279.19 cm-1 to O-H deformation and P=O stretching and 915.2 cm-1 attributed to P-OH stretching and HO–P–OH bending. In the FT-IR spectra of Urea Phosphate doped KDP crystals, same peaks with slight change in positions due to hydrogen bonding have been observed with some additional peaks. These additional peaks observed in the range 3000 cm-1 -2800 cm-1 corresponds to the C-H stretching. The peaks at around 1300cm-1 are attributed to the O-H deformation and P=O stretching, C-H Deformation, C=O and N-H Stretching. The peaks 1100cm-1 are assigned to the C-H, O-H Deformation and C-O Stretching, which confirms the doping of the crystals with Urea Phosphate.
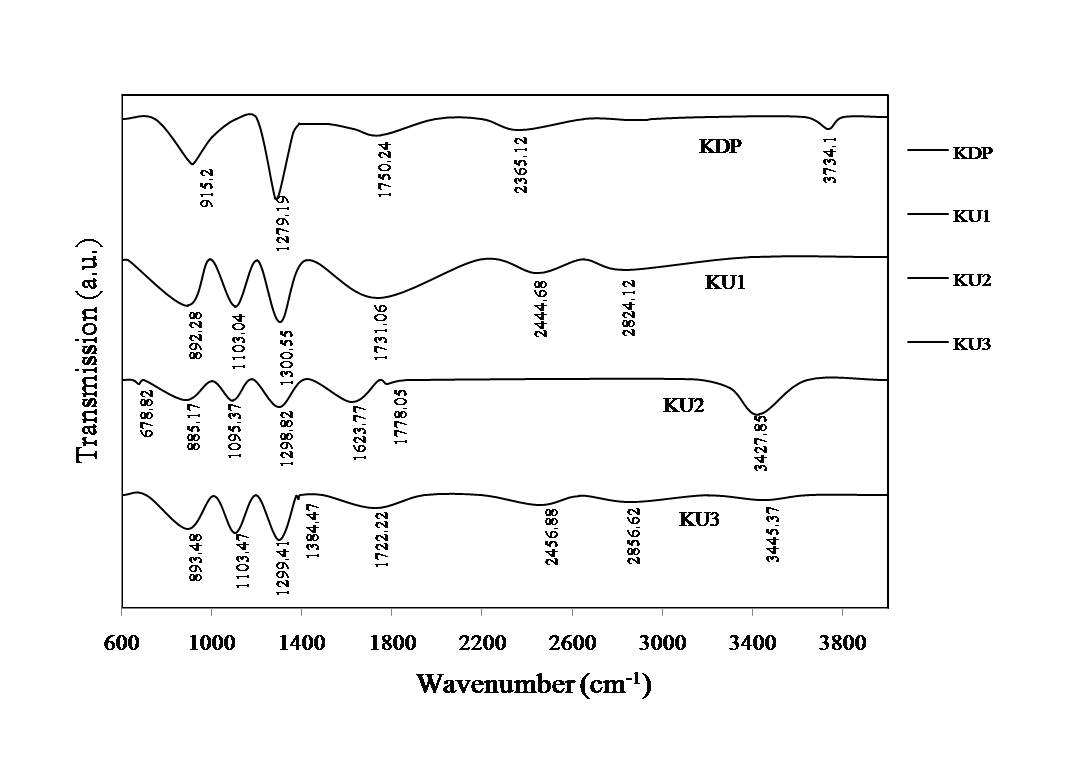 | Figure 4. FT-IR spectra of pure and doped KDP crystals |
3.3. UV-Visible-NIR Spectroscopy
- The transmission study on the grown crystal is important. The crystal should be transparent to the fundamental and second harmonic wavelength in order to have use in Second Harmonic Generation (SHG) applications. The transmission of the crystals over wavelength range 190nm-1100nm was measured on UV-1700 Shimadzu Spectrophotometer. 3mm thick crystal wafers were cut, polished and used for the transmission study. The increase in the transparency of the doped crystals has been observed. The increase in the doping level improves the fine cutoff at lower wavelength side but no change in the cutoff wavelength has been observed. UV-Vis-NIR spectrums of pure and doped KDP crystals are shown in Figure 5.
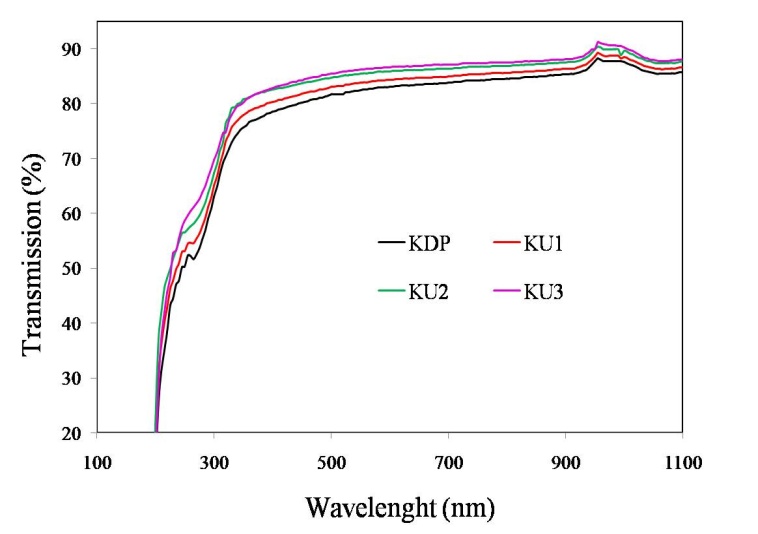 | Figure 5. UV-VIS-NIR spectra of pure and doped KDP crystals |
3.4. XRD Study
- The powder XRD patterns (Figure 6) of the pure, 6mol% UPP doped KDP crystals were recorded using X-ray Diffractometer XPERT-PRO with Cu Kα radiations (1.54060Ǻ, 40 mA, 45 kV). The powder samples were scanned in steps of 0.0170° for a time interval of 10.3359 sec over a 2θ range of 10.0144–119.9874°. The unit cell parameters have been calculated (Table 1) using software Powder X.
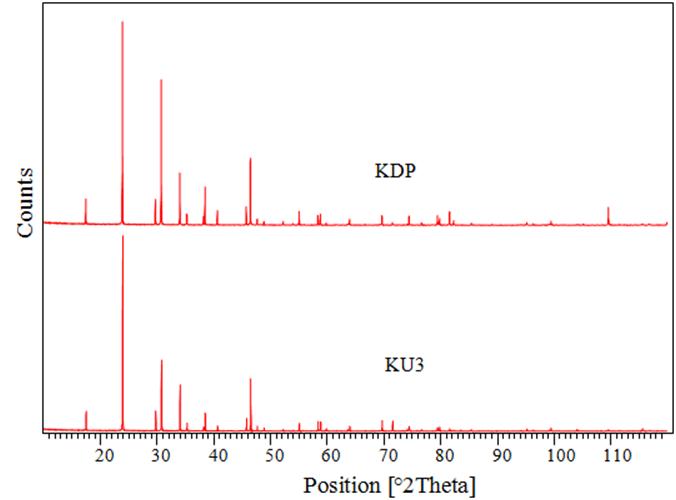 | Figure 6. Powder XRD Pattern of KDP and KU3 crystals |
|
3.5. SHG Efficiency Measurement
- Kurtz and Perry method[32] was employed to measure the SHG efficiency of the grown crystals in reference with the pure KDP. In the measurement, Q-switched, mode locked Nd:YAG laser of wavelength 1064nm of peak power 2.35mJ, pulse duration 8 ns and repetition rate 10Hz was used. The output was measured at wavelength 532nm. The SHG efficiencies of the doped crystals found to be more with reference to pure KDP (Table 2).
|
3.6. Thermal Study
- The Thermo Gravimetric Analysis (TGA) of KU3 was carried out by recording TGA curve on Perkin Elmer TGDTA at a heating rate of 15℃/min under Argon atmosphere. Pure KDP crystal is found to be stable up to temperature 235℃[13]. The TGA curve of KU3 (Figure 7) reveals the maximum weight loss is in the temperature range 195- 355℃. Prolonged heating does not produce any loss. The weight loss starts earlier and ends latter as compared to the KDP. This weight loss corresponds to the decomposition of the KDP and Urea Phosphate.
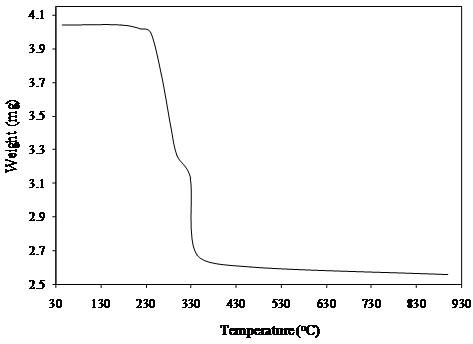 | Figure 7. TGA curve of KU3 |
4. Conclusions
- The pure and doped KDP crystals with different concentration of Urea Phosphate of best quality were grown from solution within 3-4 weeks. The doping in the crystals was qualitatively confirmed by the FTIR spectroscopy. The SHG efficiency study shows the improvement in the efficiency of the doped crystals.Increasing transparency with concentration of dopant in the host crystal has been confirmed by the UV-Vis-NIR Spectroscopy. The lattice parameters have been determined from powder XRD study. Doping in the KDP crystals leads to negligible change in the lattice parameters, and the crystal system and space group of the KDP retained.External morphology of the crystal reveals the decrease in the growth rate along[001] direction in all doped KDP crystals. The growth rate decreases with increasing doping level. This decrease in the growth rate is attributed to the attachment of the bigger size anions on pyramidal faces. The KU3 crystal is thermally stable up to 195℃.
ACKNOWLEDGEMENTS
- Author acknowledges the support of Prof. P. K. Das, IPC, IISc Bangaluru for extending the SHG efficiency measurement facility.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML