-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2012; 2(4): 81-86
doi: 10.5923/j.scit.20120204.05
Enhanced Solubility and Separation of m/p – Aminonitrobenze Using different Hydrotropic Solution
Masilamani Dhinakaran 1, Antony Bertie Morais 2, Nagarajan Nagendra Gandhi 2
1Department of Chemical Engineering, Sathyabama University, Chennai, 600 119, India
2Department of Chemical Engineering, A.C. College of Technology, Anna University, Chennai, 600 025, India
Correspondence to: Nagarajan Nagendra Gandhi , Department of Chemical Engineering, A.C. College of Technology, Anna University, Chennai, 600 025, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The aqueous solubilities of m/p – amino nitrobenzene in different concentrations (0-3.0 mol/L) of hydrotropes such as sodium benzoate, Sodium saccharin, dimethyl benzamide at different system temperatures (303K to 333K) were studied. The percentage extraction (%E) of m- amino nitrobenzene from m/p – amino nitrobenzene mixture increases with an increase in hydrotrope concentration. A Minimum Hydrotrope Concentration (MHC) in the aqueous phase was required to initiate the significance of the %E of m- amino nitrobenzene. Percentage extraction (%E) is the ratio of moles of m – amino nitrobenzene extracted in presence and absence of a hydrotrope. The sensitivity and feasibility of the proposed process are examined by carrying out solubilization and equilibrium precipitation experiments with the mixtures of various compositions. The effectiveness of hydrotropes was measured in terms of Setschenow constant Ks and reported for all hydrotropes used in this study and determination of aggregation behavior of all hydrotropes were also studied. Cost effective and eco friendly techniques of removal of less soluble compoments.
Keywords: Hydrotropy, Solubilization, Enhanced Solubility, Extraction
Article Outline
1. Introduction
- A range of industrial mixtures having a close boiling point isomeric or non isomeric components present a challenging separation problem, as in most cases conventional separation methods cannot be successfully applied. These components usually have similar chemical properties and molecular sizes and comparable volatilities. A simple technique is employed, which involves either solubilization and precipitation, i.e., the solubilization of the mixture in a hydrotrope solution and subsequent selective precipitation of a desired component by controlled dilution with water.Hydrotropy is a unique and unprecedented solubilization technique in which specific chemical components termed as hydrotropes can be used to affect a number of fold increases in the solubility of sparingly soluble solutes under standard conditions[1-4].Hydrotropic substances are a class of chemical combinations that are freely soluble in water. Hydrotropes are much effective at high concentrations which in turn enhance the aqueous solubility of organic compound, because of the opportunity of molecular solution structures probably in the form of stack-type aggregates. The solubilized solute will therefore precipitate out on dilution with water from most hydrotropic solutions. This process may be used to recover the solute in a pure form, and the remaining mother liquor may be used to concentrate the hydrotrope for recycle[5]. Even so, in modern age, we have established the aggregation behavior of common hydrotropes by several techniques[6-7]. The self-aggregation of the hydrotropes has been considered to be a pre-requisite for a number of applications in various meadows such as drug solubilization[8-10], and boswellic acids from Boswellia serrata resins[13].The current work was commenced for the fundamental study of the global character of hydrotropes in the selective separation of a component from mixtures via solubilization and precipitation techniques. With particular attention on both the theoretical understanding of the mechanistic action and the experimental studies which demonstrate the utility of hydrotropes in the separation of commercially prominent mixtures[11-17]. The system m/p – amino nitrobenzene (molecular weight M = 138.13) was selected, for enhancing its solubility using several commercially available hydrotropes. Since m- amino nitrobenzene serves as a raw material/intermediate for organic synthesis; p-phenylenediamine, azo dyes, antioxidants, fuel additives, corrosion inhibitors, pesticides, antiseptic agents, medicines for poultry and pharmaceutical synthesis and this makes its separation from any liquid mixture, which has been difficult, until now.The separation of m/p – amino nitrobenzene through solubilization and selective precipitation is important as both these isomers have been not only close boiling points but also close melting points. The melting points of m/p – amino nitrobenzene are 114℃ and 149℃ , while the boiling points are 307℃and 332 ℃, respectively. All hydrotropes are non- reactive, non-toxic and do not produce any change in temperature effect when dissolved in water. The cheapness and easy availability are other factors considered in the selection of hydrotropes.
2. Experimental
2.1. Materials
- All the chemicals used in this work were manufactured by the Loba Chemie Pvt. Ltd., Mumbai. With manufacturers stated purity of 99.9 %. The hydrotropes used in this work viz., sodium benzoate, Sodium saccharin. dimethyl benzamide are of analar grade. Double distilled water was used for the preparation of hydrotropic solutions.
2.2. Methods
- The experimental setup for conducting a single-stage batch wise liquid-liquid extraction consisted of a thermostatic bath and a separating funnel. Measurement of the solubility of m- amino nitrobenzene was carried out at temperatures of 303, 313, 323, and 333 K. For each solubility test, an equal volume (100mL) of m/p – amino nitrobenzene was comprehensively mixed to make a single-phase solution using a mechanical shaker. The hydrotrope solutions of different known concentrations were prepared by dilution with distilled water. Following to this, 100 ml of m/p – amino nitrobenzene mixture was taken and added to 100ml of hydrotrope solution of known concentration. The mixture was then made to mix consecutively for three hours. The mixture was then allowed to settle and was transferred to a separating funnel, which was immersed in a thermostatic bath with a temperature controller within ± 0.1 ℃. The setup was kept overnight for equilibration. After equilibrium was attained, the organic phase containing m- amino nitrobenzene was carefully separated and analyzed to determine the concentration using a high-performance liquid chromatography (HPLC). The mobile phase as a 40% isopropanol /60% hexane isocratic, 1ml /min and silica column is used. All the solubility trials were conducted in duplicate runs to check their reproducibility. The %E has been calculated from these solubility data. The observed error was <2%.
3. Results and Discussion
- Extracted m- amino nitrobenzene been shown in schematic comparative HPLC chromatogram in Fig.1.Experimental data on the effect of hydrotropes, i.e sodium benzoate, and Sodium saccharin and dimethyl benzamide on the percentage extractions (%E) of m- amino nitrobenzene is displayed in Figs. 2–4, and solubility of m- amino nitrobenzene is shown in Figs.5-7. Percentage extraction (%E) is the ratio of extraction of m- amino nitrobenzene in the presence and absence of hydrotrope, respectively.
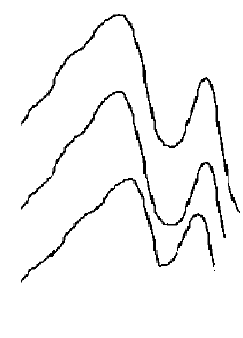 | Figure 1. Comparative HPLC chromatogram of m- amino nitrobenzene, A. Sodium saccharin ;B. sodium benzoate ; C. dimethyl benzamide |
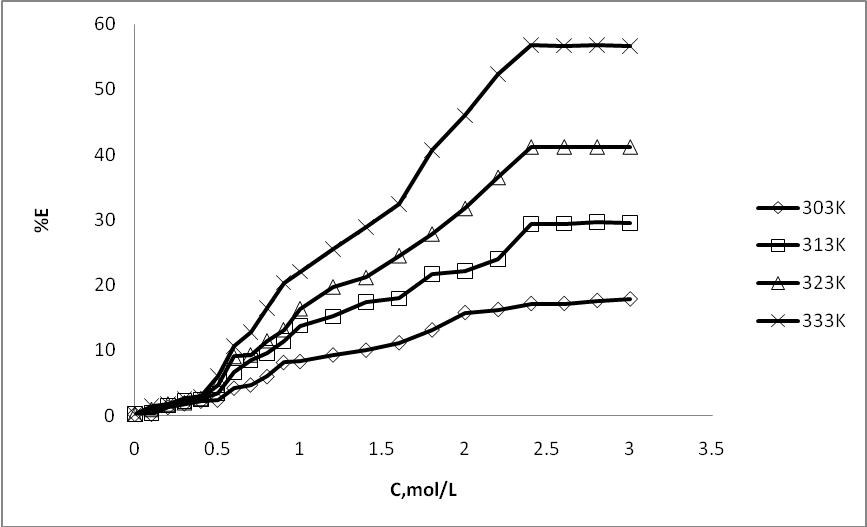 | Figure 2. Effect of Sodium saccharin concentration (C) on percentage extractions (%E) of m- amino nitrobenzene |
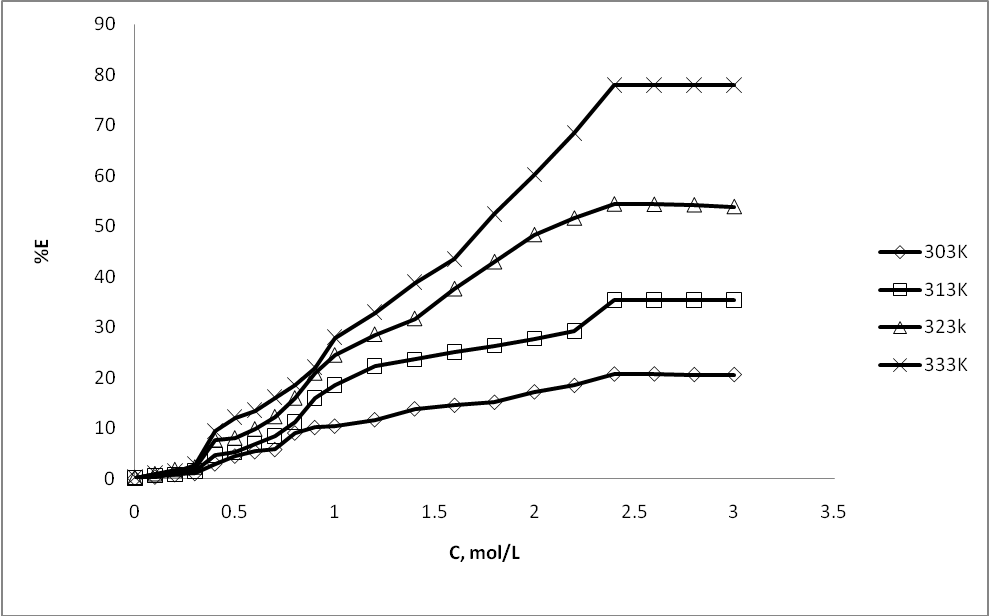 | Figure 3. Effect of sodium benzoate concentration (C) on percentage extractions (%E) of m- amino nitrobenzene |
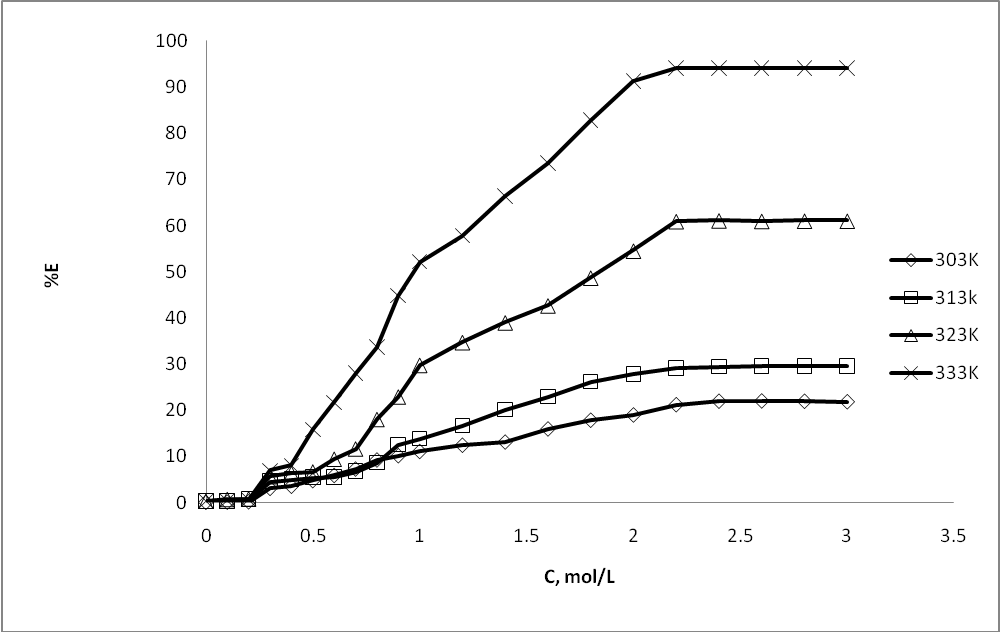 | Figure 4. Effect of dimethyl benzamide concentration (C) on percentage extractions (%E) of m- amino nitrobenzene |
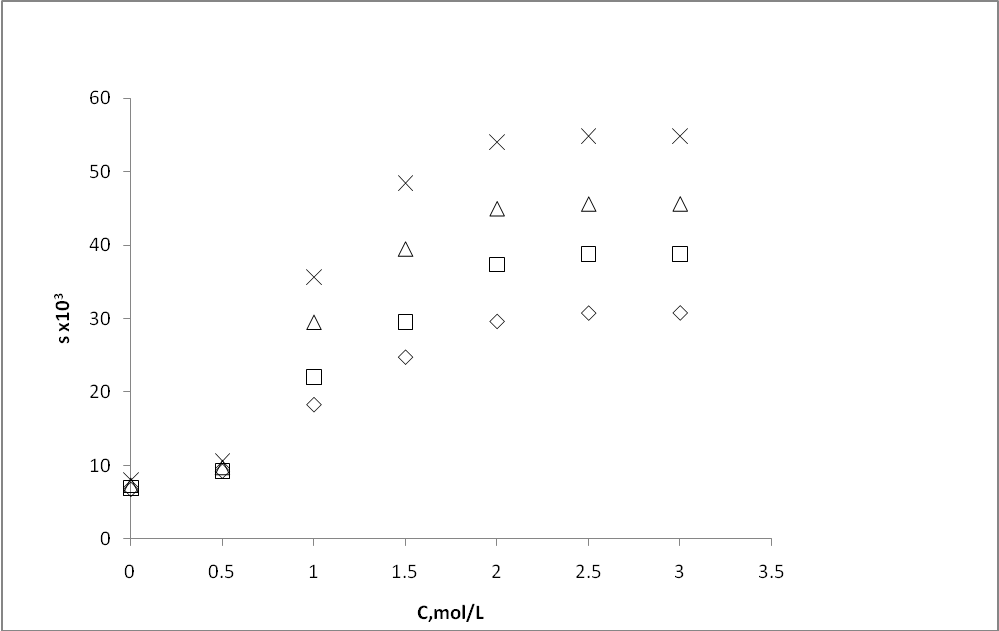 | Figure 5. Effect of Sodium saccharin concentration (C) on solubility of m- amino nitrobenzene |
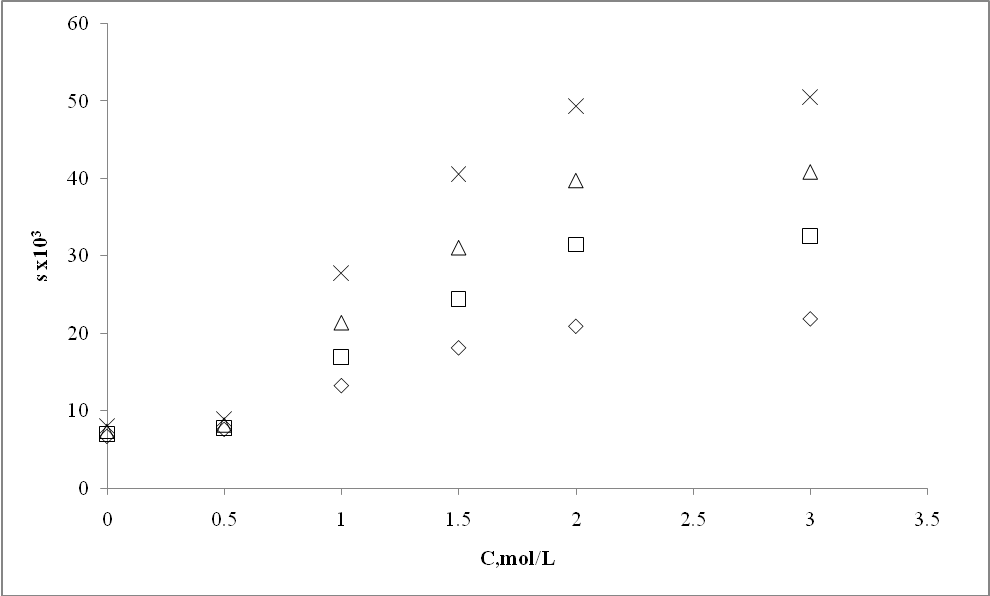 | Figure 6. Effect of sodium benzoate concentration (C) on solubility of m- amino nitrobenzene |
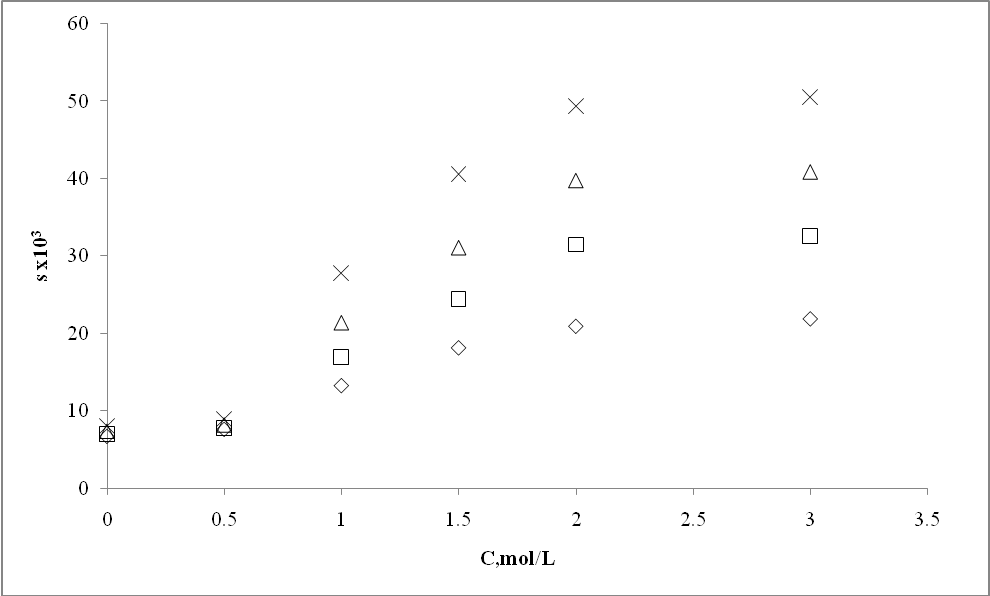 | Figure 7. Effect of dimethyl benzamide concentration (C) on solubility of m- amino nitrobenzene |
|
4. Effectiveness of Hydrotrope
- The effectiveness factor for each hydrotrope with respect to the percentage extraction of m- amino nitrobenzene at different system temperatures was determined by applying the model suggested by Setschenow and later modified by Phatak and Gaikar as given by the equation.:
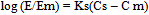 | (1) |
|
5. Association Model
- The solubility values were fitted into an association model for the hydrotropic solubilization which shows the aggregation behavior of hydrotrope and succeeding interaction of a solute with the hydrotrope assemblies. The model describes the hydrotrope-hydrotrope and hydrotrope-solute interactions with the mass-action law,
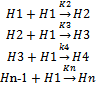 Through the hypothesis that hydrotrope molecules associate in a step-by-step manner to form oligomers and multimers such that the association constant gets to be weaker on addition of succeeding hydrotrope molecules. The association constant for an n-mer of hydrotrope with a monomer is related to the dimerization constant (K2, L/mol), i.e., Kn = K2/n.The concentration of a monomeric hydrotrope molecule,[H1], is related to the total hydrotrope concentration (CS, mol/L) through the following equations..
Through the hypothesis that hydrotrope molecules associate in a step-by-step manner to form oligomers and multimers such that the association constant gets to be weaker on addition of succeeding hydrotrope molecules. The association constant for an n-mer of hydrotrope with a monomer is related to the dimerization constant (K2, L/mol), i.e., Kn = K2/n.The concentration of a monomeric hydrotrope molecule,[H1], is related to the total hydrotrope concentration (CS, mol/L) through the following equations..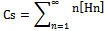 | (2) |
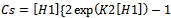 | (3) |
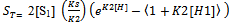 | (4) |
|
|
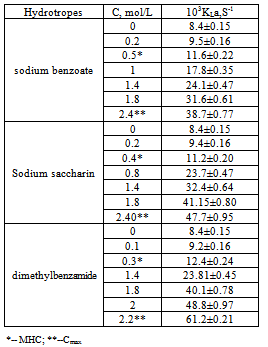
6. Mass-Transfer Coefficient
- The mass transfer coefficient of the m- amino nitrobenzene + water system in the absence of any hydrotrope is 8.4 x 10-3 s-1 at 303 K (Table 5). The effect of different hydrotropes on the mass transfer coefficient of m- amino nitrobenzene at different hydrotrope concentrations is also given in the same table. It can be seen that a threshold value of 0.30 mol/L is needed to affect significant enhancement in the mass transfer coefficient of m- amino nitrobenzene + water system, as observed in the case of solubility determinations. The mass transfer coefficient of m- amino nitrobenzene + water system increases with increase in dimethyl benzamide concentration. A similar tendency in the mass transfer coefficient of m- amino nitrobenzene has been observed for other hydrotropes also, namely, sodium benzoate and Sodium saccharin.
7. Conclusions
- Selective solubilization of isomeric combinations of m/p- amino nitrobenzene was determined in aqueous solutions of a number of hydrotropes at different hydrotrope concentrations and temperatures. The MHC and Cmax values of hydrotropes with respect to m- amino nitrobenzene can be used for the recovery of the dissolved m- amino nitrobenzene and hydrotrope solutions at any hydrotrope concentration between MHC and Cmax by simple dilution with distilled water. It was potential to extract 81% of the material, and the technique was optimized with respect to concentration of hydrotrope solution. From the data obtained by this research to be found that individual components using a step-by-step aggregation model indicated a weaker hydrotrope aggregation process but a much stronger hydrotrope–solute association. The association model predicted the trend in the solubility of the isomers, including selectivity in the solubilization of a particular isomer from the mixture. These sigmoidal-type solubility deviations are controlled by molecular structures. The differences in solubilities with hydrotrope concentration and temperature can be employed for the separation of closely related compounds. This will eliminate the huge cost and energy normally concerned in the separation of solubilized m- amino nitrobenzene from its solution. Hence dimethylbenzamide is found to be the best suitable hydrotrope for the enhancement of solubility of poorly soluble m- amino nitrobenzene within the framework of the current research.
References
| [1] | Badwan, A.-A. El-Khordagui, L.-K., Saleh, A.-M, ( 1983) The solubility of benzodiazepines in sodium salicylate solutions and a proposed mechanism for hydrotropic solubilisation International Journal of Pharmaceutions, , 13: 67-74. |
| [2] | Janakiraman, B, Sharma, M. M, (1985), Enhancing rates of multiphase reactions through hydrotropy Chemical Engineering and science. 40, 2156-2158. |
| [3] | Saleh, A.-M, El-Khordaugi, L.-K. (1985) Hydrotropic agents, International Journal of Pharmaceutions, ,24: 231- 238. |
| [4] | Balasubramanian. D, Srinivas, V, Gaikar, V.-G, (1989) Aggregation behavior of hydrotropes in aqueous solutions, Journal Physical Chemistry, ,93: 3865-3870 |
| [5] | Neuberg .c(1916), Biochem. Z., 76, 107. |
| [6] | Agarwal. M, Gaikar. V-G, (1992) Extractive separation using hydrotropes, Separations Technology, 2: 79-84. |
| [7] | Liaonanchen. X, Micheau J.-C (2002) Hydrotrope induced autocatalysis in the biphasic alkaline hydrolysis of aromatic esters, Journal of Colloid and Interface Science, 249: 172-179. |
| [8] | Lee. J, Lee. S. C, Acharya, G, (2003) Hydrotropic solubilization of paclitaxel analysis of chemical structures for hydrotropic property, Pharm. Res, 20(7): 1022-1030 . |
| [9] | Maheshwari.R.K, Deswal.s, Tiwari.D, Ali.N (2008), Analysis of amoxicillin by application of hydrotropic solubilization phenomenon in solid dosage ,Asian J. Chem.,20,(1), 805-807. |
| [10] | Maheshwari.R.K, (2006) A novel application of hydrotropic solubilization in the analysis of bulk samples of ketoprofen and salicylic acid, Asian J. Chem, ,18,(1), 393-396. |
| [11] | Raman.G , Gaikar,V.G, (2003),Hydrotropic solubilization of boswellic acids from boswellia serrata resin, Langmuir, 19, 8026-8032. |
| [12] | Nagendra Gandhi. N , Dharmendira Kumar. M. (2000), Effect of Hydrotropes on Solubility and Mass Transfer Coefficient of Methyl Salicylate. J. Chem. Eng. Data 45, 419–423. |
| [13] | Nagendra Gandhi.N, Dharmendira Kumar.M, N Sathyamurthy. N, (1998),Effect of Hydrotropes on Solubility and Mass-Transfer Coefficient of Butyl Acetate J. Chem. Eng. Data.,43: 695-699. |
| [14] | Nagendra Gandhi .N, Dharmendira Kumar. M , Sathyamurthy.N , (1998) Solubility and Mass Transfer Coefficient Enhancement of Ethyl Benzoate through Hydrotropy, Hungarian Journal of industrial chemistry, , 26, 63-68. |
| [15] | Dhinakaran.M, Antony Bertie Morais, and Nagendra Gandhi.N, (2012) Influence of Hydrotrop on Solubility And Mass Transfer Co Efficient Enhancement ofTriphenylcarbinole ,R.J. chem.Sci.,2(5),35-41, |
| [16] | Dhinakaran.M Antony bertie morais and Nagendra Gandhi.M, (2012) Separation of m/p-Aminoacetophenone Using Hydrotropy , E-J Chem. 9(4), 2006-201 |
| [17] | Dhinakaran.M, Antony bertie morais and Nagendra Gandhi.M, (2011) separation of 2, 4 / 2,6 - xylidine mixture through hydrotropy, oriental journal of chemistry, 27, (4): 1671-167 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML