-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2012; 2(4): 61-65
doi: 10.5923/j.scit.20120204.02
Conformational Analysis of Humic Acids from Amazonian Dark Earth Soils observed by AFM
F. L. Leite 1, D. K. Deda 1, M. L. Simões 2, W. T. L. da Silva 2, L. Martin-Neto 2, T. J. F. da Cunha 3
1University of São Carlos (UFSCar), 18052-780, Sorocaba -SP, Brazil
2Embrapa Agricultural Instrumentation, National Nanotechnology Laboratory for Agriculture, P. O. Box 741, 13560-970, São Carlos, SP, Brazil
3Embrapa Tropical Semi-Arid Center (CPATSA), BR 428, Km 152, Zona Rural, P. O. Box 23, 56302970, Petrolina-PE, Brazil
Correspondence to: F. L. Leite , University of São Carlos (UFSCar), 18052-780, Sorocaba -SP, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Conformational analysis of humic acid (HA), extracted from an Amazon anthropogenic soil and an adjacent soil which did not have anthropogenic A horizon, was done by atomic force microscopy (AFM), through the deposition of humic acid layers on muscovite mica strips using drop-casting deposition technique. This short communication presents, for the first time, AFM images of the sub-micron level structure of humic acid of the Amazonian Dark Earths, compared to natural pedogenic Amazonian soil. The adsorbed anthropogenic HA form ring-shaped aggregates with diameters on the scale of several tens of nanometers, possibly showing evidences of a supramolecular formation. The formation of these structures was not verified for HA from pedogenic soil. In this case, it was observed particles with globular shape and a homogenous distribution of them on the mica surface. Structural characteristics of each sample were observed by nuclear resonance magnetic spectroscopy (NMR). The spectra analysis indicated that pedogenic HA are richer in aliphatic groups, as methoxylic and in polysaccharides structures, while the higher concentrations of aromatic carbon, including also phenolic carbon, were observed in the anthropogenic HA, justifying its higher hydrophobic character.
Keywords: Humic Acid, Atomic Force Microscopy, Anthropogenic Soil, Supramolecular Structures, Amazon Dark Earth, Nuclear Resonance Magnetic
Article Outline
1. Introduction
- Soil Organic Matter (SOM) play an important role on many aspects of the nature of soil and environmental processes. Humic Substances (HS) are the major organic constituents of soils and aquatic environments and are generated by the microbiological and chemical degradation, and transformation of organic matter (OM), resulting in chemical structures which are more stable than the starting material[1-4]. Most of the soils in the Amazon Basin are acidic, with low cation-exchange capacity, fertility and production potential. In this environment, where soil fertility is a limiting factor for sustainable agricultural development, occurs the “Terra Preta de Índio” soil[5], also known as Amazonian Dark Earth, Anthropogenic Dark Earth, Indian Black Earths, or Archaeological Dark Earths. These soils, that have an archeo-anthropedogenic horizon, which is a surface horizon with variable depth in soil profiles, exhibit elevated OM contents and either ceramic pieces or lithic artifacts[6]. The OM present in these soils shows high stability and reactivity characteristics[7]. The origin of this OM is thought to bemostly pyrogenic and is related to the domestic/agricultural activity of pre-columbian populations that colonized the Amazon basin since 3000 B.C.[8]. Due to its high fertility, compared to the adjacent soils, it is has been frequently used in subsistence agriculture (beans, corn, etc.) and/or for commercial production of a high diversity of products (papaya, coconut, cupuaçu etc.). The chemical nature and reactivity of HS are still little understood. Several researchers[9-11] have suggested a new model for structure of HS, in which small and heterogeneous humic molecules are self-assembled in supramolecular conformations stabilized only by weak forces. Several techniques have been employed to characterize the size, shape, conformation, structure and composition of HS. Fluorescence spectroscopy, for example, has become widely recognized as a relatively simple, sensitive, and powerful tool for the evaluation of humification[12,13] and the molecular size of humic substances[14,15]. Nuclear Ressonance Magnetic also is commonly used to characterize complex HS including humic and fulvic acids[16-19]. In addition, other techniques such as capillary electrophoresis[20], ultraviolet and infrared spectroscopy[21,22] and scanning electron microscopy (SEM)[23] have also been frequently employed.Atomic Force Microscopy (AFM) technique can image surfaces with atomic resolution by scanning a sharp tip across the surface at forces smaller than the forces between atoms[24]. AFM has been employed to study the morphologies of humic and fulvic acid[25-28] and it is a powerful tool to characterize small colloids, as well as colloid agglomeration, adsorption onto surfaces, or modification in the morphologies, affected by changes in the physical-chemistry properties. Namjesnik-Dejanovic and Maurice[29] reported that high or low concentrations of fulvic acids, from river water, result in different structures, which can be differentiated by AFM. Chen and coworkers[30] combined AFM with SEM to investigated macromolecular structures of humic substancesThey reported that, depending on the pH and ionic strength of the initial solutions, humic substances deposited on mica and silica, adopted different spherical and large-area island conformations.In a general way, a better understanding of HS structures will provide information of origin and genesis, reactivity, and role in environmental processes. The objective of this work was to study the anthropogenic soil humic acid in comparison to an adjacent soil which did not have anthropogenic A horizon, using AFM and, complementally, NMR. The purpose of our investigation was to obtain new data of morphology, adsorption and orientation of HA structures on mica from anthropogenic HA and shows evidences from supramolecular formation for the first time in Amazonian Dark Earth.
2. Material and Methods
- Humic acids (HA) from two different origins, pedogenic and anthropogenic soil, both obtained from the Amazon State (Brazil), were extracted and purified according to the International Humic Substances Society (IHSS) method[31]. Suspensions of 100 mg L-1 of HA from anthropogenic Dark Earths and adjacent soil, were prepared suspending HA samples in deionized water. The suspension was shaken for at least three days. The pH was adjusted to 3.5 using HCl. Muscovite mica with a surface area of about 1 cm2 was cleaved and kept into a Beaker containing 10 ml of a 100 mg L-1 HA suspension for 1 day, under constant stirring. The mica was then rinsed with deionized water, put in a Petri dish and kept in a diseccator for 12 hours before obtaining the AFM images[32]. Unrinsed samples were also analyzed and used as comparison.NMR experiments were performed with a Varian model Unity Inova 400 Spectrometer (13C resonance frequency of 100.58 MHz), equipped with a solid Doty Supersonic probe. The HA samples (approximately 300 mg) were conditioned in a zirconium cylindrical rotor with 5 mm of external diameter (Doty Supersonic). The variable amplitude cross polarization magic angle spinning technique (VACP-MAS 13C NMR)[33] was applied with a contact time of 1 ms, acquisition time of 12.8 ms, pulse delay of 500 ms, spinning speed of 6 kHz. The 13C chemical shifts were referenced using hexametylbenzene − the resonance line in 17.2 ppm.AFM imaging were obtained at room temperature, using an Atomic Force Microscope TMX 2010 Topometrix, including special silicon nitride (Si3N4) cantilevers with spring constant of 0,032 N m-1 and pyramidal tip with radius of 40 nm. To investigate the samples with AFM, it was used the contact mode tip and scan rate of 1.5 Hz. Surface scans of AFM were analyzed using a freeware scanning probe microscopy software based on MS-Windows named WSXM[34].
3. Results and Discussions
- A typical image of pedogenic HA adsorbed on mica surface is shown in Figure 1a. The globular and cone-shaped features were assigned to islands of HA adsorbed on mica surface, and were identified two general types of structures, which can be summarized as: particles or spheroids and network of particles. AFM images show that large aggregates and small particles coexisted. The pH of HA suspension (3.5) favors the aggregate conformation due to a shielding of charges of functional groups and, under these conditions, intermolecular interactions between the particles are also favored[35].
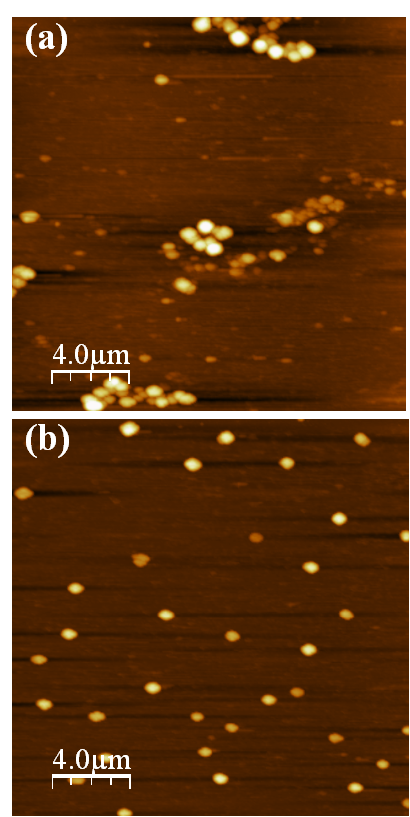 | Figure 1. Contact mode AFM images (2020 μm) of pedogenic humic acid (pH=3.5) adsorbed on mica surface. (a) unrinsed and (b) rinsed |
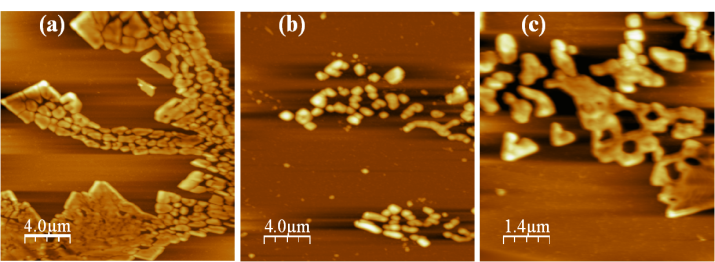 | Figure 2. Contact mode AFM image (2020 μm) of anthropogenic humic acid (a) unrinsed, (b) rinsed and (c) zoom-in from Figure 2a showing ring-type structures (77 μm) |
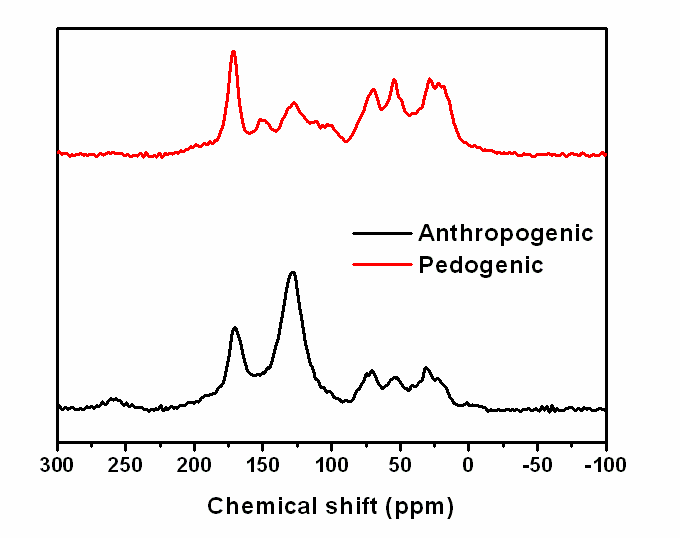 | Figure 3. VACP-MAS 13C NMR spectra of the pedogenic and anthropogenic humic acids |
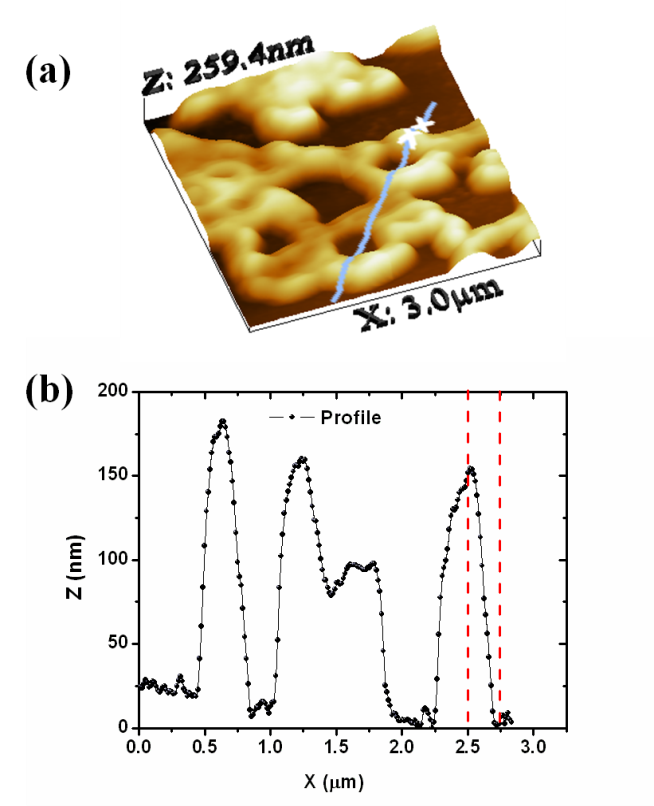 | Figure 4. (a) 3-D perspective view of a ring-type structure (33 μm) and (b) result of section analysis for humic acid adsorbed on mica |
4. Conclusions
- AFM was successfully used to measure HA adsorption on a mica surface. The results gave evidence that structure formation in thin HA films can be caused by dewetting during film deposition, and solution physical-chemistry. Under the experimental conditions used in this study, the adsorbed molecules form ring-shaped aggregates with diameters on the scale of several tens of nanometers; smaller nanometer-scale rings present along the circumference could potentially represent hydrophobic domains. The different shapes of HA under different conditions suggest a supramolecular structure. The AFM data of the vertical dimension suggest smaller colloids; this might be due to a size selectivity of the adhesive forces and the attachment process to the mica, favoring smaller particles. The data of the lateral dimension shows a larger spread (up to several 100 nm), which cannot be explained by the tip artifact (∼40 nm). Additional studies to determine the changes in the HA structures as a function of solution conditions, is necessary. Other techniques such as small-angle X-ray scattering (SAXS), scanning electron microscopy (SEM) and molecular dynamic may provide information important on HA of the Amazonian Dark Earths and already are being performed.
ACKNOWLEDGEMENTS
- This work was supported by FAPESP, FINEP, CAPES and CNPq.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML