-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2012; 2(3): 50-54
doi: 10.5923/j.scit.20120203.04
Experimental Study of the Thermophysical and Acoustical Properties of Acetophenone and Propylacetate
K. Saravanakumar 1, T. R. Kubendran 2
1Department of Chemical Engineering, Sathyabama University, Jeppiar Nagar, Chennai, 600119, India
2Department of Chemical Engineering, A.C. College of Technology, Anna University, Chennai, 600025, India
Correspondence to: K. Saravanakumar , Department of Chemical Engineering, Sathyabama University, Jeppiar Nagar, Chennai, 600119, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
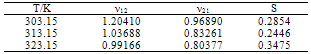
Densities, viscosities, refractive indices and ultrasonic velocities of the binary mixtures of Acetophenone with Propyl acetate were measured over the entire mole fractions at (303.15, 313.15 and 323.15) K. From these experimental results, excess molar volumes VE, viscosity deviation ∆η, refractive index deviation ∆nD, deviations in isentropic compressibility ∆Ks and excess intermolecular free length ∆Lf are calculated. The viscosity values were fitted to the models of Krishnan- Laddha and McAllister. The thermo physical properties under study were fit to the Jouyban - Acree model. The excess values were correlated using Redlich-Kister polynomial equation to obtain their coefficients and standard deviations. It was found that in all cases, the data obtained fitted with the values correlated by the corresponding models very well. The results are interpreted in terms of molecular interactions occurring in the solution.
Keywords: Viscosity, Density, Refractive Index, Ultrasonic Velocity, Molecular Interactions
Article Outline
1. Introduction
- The thermodynamic, acoustic and transport properties of liquids and liquid mixtures[1] are used to study the molecular interactions between the various components of the mixtures and also to understand engineering applications concerning heat transfer, mass transfer, and fluid flow. In chemical process industries, materials are normally handled in fluid form, and as a consequence, the physical, chemical, and transport properties of fluids, assume importance. Thus, data on some of the properties associated with the liquids and liquid mixtures like density, viscosity, refractive index and ultrasonic velocity find extensive application in solution theory and molecular dynamics[2]. Such results are necessary for interpretation of data obtained from thermo chemical, electrochemical, biochemical and kinetic studies[3]. Acetophenone is an important industrial chemical widely used as an ingredient of flavour and fragrance in soaps, detergents, cosmetics and perfumes. Propyl acetate is used as a solvent in inks for coatings, cosmetics and personal-care products, intermediate for pharmaceuticals & agrochemicals, flexographic and rotogravure printing. Acetophenone and propyl acetate mixture was used in the manufacture of cosmetics amd fruit flavouring agents. In our earlier paper, we had studied the transport properties of binary liquid mixtures[4,5]. In continuation of this research, we have reported density (ρ), viscosity (η), refractive index (nD) and sound speed (u) of pure acetophenone and propyl acetate for the binary system constituted by these two chemicals at temperatures of 303.15, 313.15 and 323.15 K. The viscosity values have been fitted to McAllister[6] and Krishnan and Laddha[7] model. The Jouyban –Acree[8] model has also been extended to density, viscosity, refractive index and sound speed (u) of binary mixtures. The deviation values have been fitted to Redlich-Kister[9] equation. Literature survey showed that no measurements have been previously reported for the mixture studied in this paper.
2. Materials and Methods
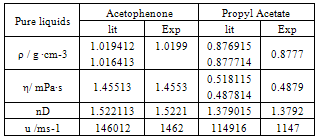
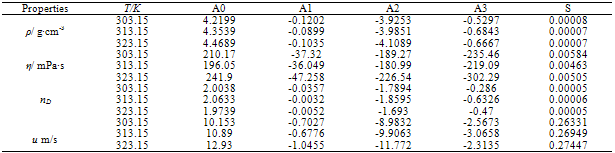
- All the chemicals used in this study were of analytical grade and obtained from Lobo Chemicals, India. The claimed mass fraction purity for the chemicals was ≥0.998. These chemicals were dried over molecular sieves and partially degassed prior to use[10, 11]. The purity of these experimental chemicals was checked by comparing the observed densities, viscosities, refractive indices and velocities with those reported in the literature. The measured values are included in Table 1 along with the available literature values.Binary mixtures are prepared by mixing appropriate volumes of the liquid components in the specially designed glass bottles with air tight Teflon coated caps and mass measurements performed on a Shimadzu Corporation Japan type BL 2205 electronic balance, with a precision of ±0.01 mg. The required properties are measured on the same day immediately after preparing each composition. For all measurements, temperatures were controlled by circulating the water through a thermostat (Technico,Madras. made in India) keeping temperature fluctuations within ±0.03K.
|
2.1. Density
- Densities were determined by using a 25 cm3 bicapillary pycnometer and calibrated with deionized double distilled water with a density of 996.0 kg ·m-3 at a temperature of 303.15 K. The pycnometer was thermostatted in a transparent walled water bath (maintained constant to ± 0.01 K) for 15 min to attain thermal equilibrium, and the liquid level in the two arms was obtained with a traveling microscope which could read to 0.01 mm. The precision of the density measurements was estimated to be ± 0.0003 g ·cm-3.
2.2. Kinematic Viscosity
- The kinematic viscosities were measured with Ostwald viscometer previously calibrated using water. The time was measured with a precision of 0.01s, and the uncertainty in the viscosity was estimated to be less than 0.0003 mPa·s. The kinematic viscosity was obtained from the working equation
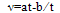 | (1) |
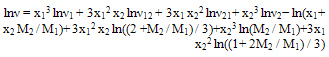 | (2) |
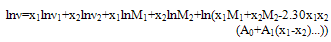 | (3) |
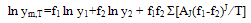 | (4) |
2.3. Refractive Index
- Refractive indices were measured using thermostatically controlled Abbe refractometer (Atago 3T) with accuracy less than 0.001units. Water was circulated in to the prism of the refractometer by a circulation pump connected to an external thermo stated water bath. Calibration was performed by measuring the refractive indices of doubly distilled water and propyl alcohol at defined temperatures. The sample mixture was directly injected in to the prism assembly of the instrument using a syringe. The solutions were pre thermo stated at the temperature of the experience before the experiments to achieve a quick thermal equilibrium.
2.4. Sound Speed
- Speed of sound was measured by using a variable path, single crystal interferometer (Mittal Enterprises, New Delhi) at a frequency of 2MHz. The interferometer was calibrated using toluene. The interferometer cell was filled with the test liquid, and the temperature of the solution was maintained constant within ±0.01 K by circulation of water from a thermostatically regulated water bath through the water jacketed cell. The uncertainty was estimated to be 2 ms-1. The isentropic compressibility was calculated by the equation
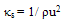 | (5) |
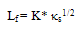 | (6) |
3. Results and Discussion
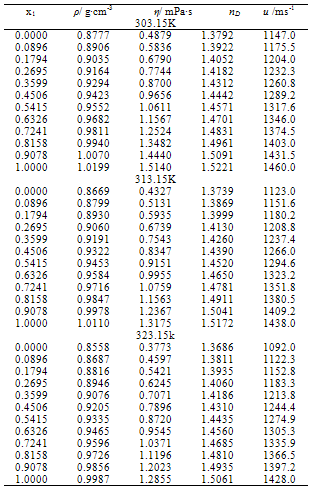
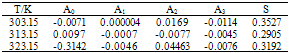
- Measured values of densities, viscosities, refractive indices and ultra sonic velocities of acetophenone with propyl acetate at temperatures of (303.15, 313.15, and 323.15) K are listed in Table 2.The density values have been used to calculate excess molar volumes VE using the following equation
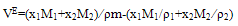 | (7) |
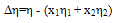 | (8) |
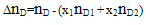 | (9) |
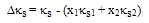 | (10) |
|
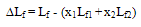 | (11) |
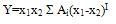 | (12) |
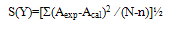 | (13) |
|
|
|
|
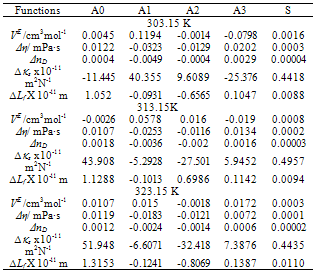
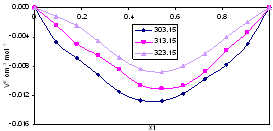 | Figure 1. Excess Molar Volume, VE, for the system Acetophenone (1) + Propyl Acetate (2) at temperatures: ♦, T= 303.15 K; ■, T= 313.15 K; ▲, T= 323.15 K |
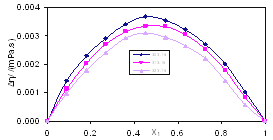 | Figure 2. Viscosity Deviation, Δη, for the system Acetophenone (1) + Propyl Acetate (2) at temperatures: ♦, T= 303.15 K; ■, T= 313.15 K; ▲, T= 323.15 K |
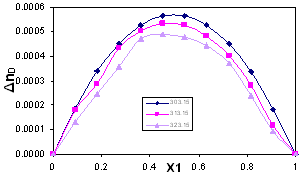 | Figure 3. Refractive Index Deviation, ΔnD , for the system Acetophenone (1) + Propyl Acetate (2) at temperatures: ♦, T= 303.15 K; ■,T= 313.15 K; ▲,T= 323.15 K |
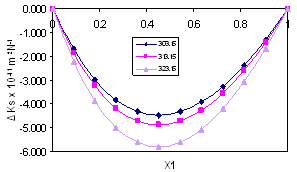 | Figure 4. Isentropic Compressibility Deviation, ∆κs , for the system Acetophenone (1) + Propyl Acetate (2) at temperatures: ♦, T= 303.15 K; ■,T= 313.15 K; ▲,T= 323.15 K |
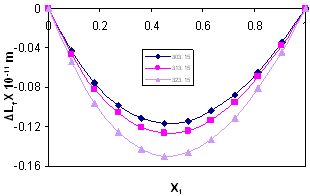 | Figure 5. Intermolecular Free Length Deviation, ∆Lf , for the system Acetophenone (1) + Propyl Acetate (2) at temperatures: ♦, T= 303.15 K; ■,T= 313.15 K; ▲,T= 323.15 K |
4. Conclusions
- Densities, viscosities, refractive indices and ultrasonic velocities for a four binary mixtures have been measured. Excess molar volumes, viscosity deviations, refractive index deviations, compressibility deviation and change in intermolecular free length for mixtures of acetophenone and propyl acetate were obtained from the experimental results and fitted by the Redlich Kister equations. It has been concluded that the Jouyban Acree model is very well suited for correlating the thermo physical properties of the binary mixture studied.
ACKNOWLEDGEMENTS
- The authors thank the University authorities for providing the necessary facilities to carry out the work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML