-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2012; 2(1): 51-52
doi: 10.5923/j.scit.20120201.10
Solubility and Solvation Parameters of Calcium Carbonate in Mixed Ethanol-water Mixtures at 301.15 K
Esam A. Gomaa
Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt
Correspondence to: Esam A. Gomaa , Chemistry Department, Faculty of Science, Mansoura University, Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The molar solubility of calcium carbonate (CC) in mixed ethanol (ETOH)-water solvents was measured at 301.15 K. From the molar solubilities, the solvation parameters, activity coefficients, solubility products, free energies of solvation and transfer free energies for interaction of (CC) from water as reference solvent to mixed (EtOH-H2O) solvents were evaluated. All the salvation parameters were discussed.
Keywords: Molar Solubility, Calcium Carbonate, Free Energies of Solvation, Water , Ethanol
Cite this paper: Esam A. Gomaa , "Solubility and Solvation Parameters of Calcium Carbonate in Mixed Ethanol-water Mixtures at 301.15 K", Science and Technology, Vol. 2 No. 1, 2012, pp. 51-52. doi: 10.5923/j.scit.20120201.10.
1. Introduction
- The solubility of solutes in mixed solvents is of great practical importance since many industrial process as well as laboratory procedures call for the use of solvent mixtures. The solubility of solutes in mixed solvents depends primarily on the solvation of solutes or their constituent ions by the components of solvent mixtures[1]. Studying the thermodynamics of different salts, is important for evaluating the single ion thermodynamic parameters which help in explain the preferential solvation of the ions[2].Removal of heavy elements and sulphate ions from an alkaline medium using solvent extraction was very important to get rid of these hard ions[3].
2. Experimental
- The used calcium carbonate (CC) and ethanol (EtOH) were supplied from Merck Co. The saturated solution of calcium carbonate (CC) was prepared by dissolving little solid amount in closed test tubes containing different EtOH-H2O mixtures. The mixtures were then saturated with nitrogen gas as inert atmosphere. The tubes were placed in a shaking thermostat (Model GEL) for a period of one week till equilibrium reached.The solubility of CC in each mixture were measured conductmetrically exactly (three times minimum) by using conductmeter of the type YSI model-35 and it was connected with an ultra-thermostat of the type Kottermann-4130. All conductance were measured at 301.15 K. The accuracy of the solubility data is in average of third number aftercoma, as in previous work[4].
3. Results and Discussion
- The molar solubility for calcium carbonate (CC) at 301.15 K were measured conductmetrically and the –log S values are cited in Table water, ethanol (EtOH) and their mixtures. The solubility of (CC) in water agreed well with that in literature[5].The activity coefficients were calculated by the use of Debye-Hückel equation[6].
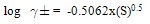 | (1) |
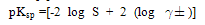 | (2) |
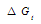 increase in positivity by increasing the mole fraction of ethanol in the mixtures. This is due to more difficult solvation in the mixed solvents than that of water. Polar solvents like H2O or EtOH or their mixtures cannot penetrate (CC) lattice due to the very high heats of formation, free energy, entropy and heat capacity for (CC) salts[7], calcite and aragonite.
increase in positivity by increasing the mole fraction of ethanol in the mixtures. This is due to more difficult solvation in the mixed solvents than that of water. Polar solvents like H2O or EtOH or their mixtures cannot penetrate (CC) lattice due to the very high heats of formation, free energy, entropy and heat capacity for (CC) salts[7], calcite and aragonite.
|
|
References
| [1] | Yizhak Marcus, "Solubility and solvation in mixed solvent systems", Pure and Applied Chem., 62 (1990) 2069-2076 |
| [2] | Esam A. Gomaa, "Single ion free energies of some ion and the hydrophobic interactions of Ph4 AsBPh4 and Ph4SbB Ph4 in mixed ethanol-water solvents". Thermochimica Acta, 156 (1989) 91-99 |
| [3] | Cleophase Ngoie Mpinga, "Removal of aluminium and sulphate ions from alkaline medium using solvent extraction”., Master of Technology, Faculty of Engineering, Cape Peninsula University of Technology (2009) |
| [4] | E. A. Gomaa, "Solvation parameters of lead acetate in mixed water-N, N-Dimethylformamide mixtures at 298.15 K ". Analele Universitätii den Bucuresti, 19, 1 (2010) 45-48 |
| [5] | Perry's "Chemical Engineering Handbook, Section 2, Physical and Chemical data, 8th Edition, McGraw Hill., USA (2008) |
| [6] | A. A. El-Khouly, Esam A. Gomaa and S Abou El-Leef, "Conductometry and solubility study of Cd2+ Kryptofix-221 complexes in various hydroorganic solvents", Bulletin of Electrochemistry, 19(4), (2003) 153-164 |
| [7] | Jim Plambec la @ Malberta. Ca |
| [8] | L. Vicum, M. Hazzotti and J. Baldyga, "Applying a thermodynamic model to the non-stoichiometric precipitation of barium sulphate". Chemical Engineering and Technology, 26(2003) 352-333 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML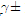 ), solubility products (pksp) and salvation free energies (
), solubility products (pksp) and salvation free energies (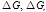 ) for molar solubilities, and free energies CaCO3 (CC) in different ethanol-water mixtures at 301.15 K
) for molar solubilities, and free energies CaCO3 (CC) in different ethanol-water mixtures at 301.15 K