-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Science and Technology
p-ISSN: 2163-2669 e-ISSN: 2163-2677
2011; 1(1): 29-33
doi: 10.5923/j.scit.20110101.05
Supercritical Carbon Dioxide Extraction of Indian Orange Peel Oil and Hydro Distillation Comparison on Their Compositions
Omprakash H. Nautiyal , Krishan Kant Tiwari
Department of Chemical Engineering, Institute of Chemical Technology, NM Parikh Marg, Matunga (E), Mumbai, 400019, India
Correspondence to: Omprakash H. Nautiyal , Department of Chemical Engineering, Institute of Chemical Technology, NM Parikh Marg, Matunga (E), Mumbai, 400019, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Orange peel oil being very useful in foods, flavor, and pharmaceutical industry consumed worldwide. An attempt was made to extract the oil using SC-CO2 to study the quality, quantity and compositions of the oil. Pressures ranging from 8-15 MPa with temperatures ranging from 28-60℃ were employed. Quality of the oil was analyzed by GC and Capillary GC. Supercritical carbon dioxide was found to extract the oil containing all the low volatile fractions and oil obtained was light pale yellow in color. Shelf life of the oil was found much better than that extracted with conventional techniques. Various pre-treatments were exercised to prolong the good life of peels to study the impact on their physical properties and quality along with the yields. Alkaline treatment of the orange peels reveals some important observations in the extractions of constituents.
Keywords: SC-CO2, Orange Peels, Pre Treatments, Hydro Distillation, Quality, Yield
Cite this paper: Omprakash H. Nautiyal , Krishan Kant Tiwari , "Supercritical Carbon Dioxide Extraction of Indian Orange Peel Oil and Hydro Distillation Comparison on Their Compositions", Science and Technology, Vol. 1 No. 1, 2011, pp. 29-33. doi: 10.5923/j.scit.20110101.05.
Article Outline
1. Introduction
- Our aim of the research was to develop the feasible process of extracting Orange peel oil and to best manage the huge waste of orange peels. Different range of pressure and temperature was studied along with batch time variation. Flow rate of supercritical carbon dioxide was not of great importance as the higher flow rates did not contributed in the yield enhancement.Hence the study was investigated with the flow rate, 5 kg h-1 and resulted in recovering the Orange oil with the best yield and good flavor value. Researchers conducted by various authors using SC-CO2 were found to show the interesting results but our study has obtained the best yield of the oil than that reported.[1-5]Supercritical fluid (SCF) extraction is an extraction process utilizing a fluid as an extract -ant at temperatures and pressures exceeding its critical temperature and pressure. Within the past three decades numerous industrial and academic research and development laboratories have investigated the underlying fundamentals and process applications of supercritical fluid (SCF) as solvents. It is possible to separate a multi component mixture when a supercritical fluid is used as an extractive solvent bycapitalizing on both the differences in component volatilities (i.e., salient features of distillation) and the differences in the specific interaction between the mixture components and the SCF solvent (i.e., the salient features of solvent extraction). The application of SCF solvents is based on the experimental observations that many gases exhibit enhanced solvating power when compressed to conditions above the critical point. G. Della Porta1996 have studied desorption of bigarade peel oil from a polar adsorbent was performed by supercritical CO2 to improve the oil quality by selectively eliminating hydrocarbon terpenes and coumarins. The oil fractions obtained at 40°C, at pressures between 7.7 and 12MPa, and at different desorption times were analyzed by GC−MS. Three fractions were characterized: terpenic fraction, deterpenated fraction, and residue. The content of oxygenated compounds in the deterpenated fraction was 6.6 times higher than in the crude oil. Coumarins, psoralens, polymethoxy flavones, and waxes contained in the starting material were recovered and identified in the residue fraction. Gonzalo A. N. et al. 2010 studied the solubility in supercritical carbon dioxide (CO2) of farnesol using a static-analytic method (a high-pressure static equilibrium cell coupled to an HPLC). The differences in solubility between farnesol, naringenin, and other sesquisterpenes or flavonoids reported in the literature were partially explained by differences in molecular weight and polarity between solutes. They correlated experimental data as a function ofthe system temperature and pressure and the density of the solvent using a literature model that also showed the auto consistency of the data for CO2 densities above 412 kg·m−3 for naringenin.[5-8]B. Mira, M. Belasco, S. Subirats and A. Berna 1996 studied the supercritical fluid extraction of orange essential using dehydrated orange peel (0.0538 kg H2O kg−1 dm) from nave line cultivars as raw material and CO2 as solvent. The effect of operation conditions was analyzed in a series of experiments at 313 and 323K and pressures between 1 and 25 MPa. Furthermore, the effect of CO2 flow rate and particle size of orange peel was studied in the range of 0.5 to 3.5 kg h−1 and 0.1 to 10 mm. The subcritical CO2 dissolves hardly any essential oils, however, on reaching the critical point, the amount of essential oils dissolved increased with pressure, within the range of pressures considered in this study. Increasing solvent mass flow decreased the extraction efficiency while increasing particle size of orange peel decreased the extraction rate. For a rapid extraction, particle sizes lower than 2 mm and solvent mass flows lower than 2.5 kg h−1 are adequate. A model based on the assumption of plug flow of a supercritical solvent through a fixed bed of milled material was applied to analyze the experimental results. The collected extracts were orange in color due to the carotenoid and flavonoid content. During the process, water was extracted in the final stage of the extraction.[9-11]
2. Materials and Methods
2.1. SC-CO2 Extraction
- Carbon dioxide gas (99.9%) was purchased from Indian Oxygen Limited. Orange peels were collected freshly from fruit merchant, local area of Matunga, Mumbai suburbs. The standards orange oil constituents were purchased from Sigma-Aldrich Co. (St. Louis, MO). The SC-CO2 pilot plant (Figure 1), with extractor and separator capacities of 1 L each, was imported from UHDE GmbH (Dortmund, Germany).
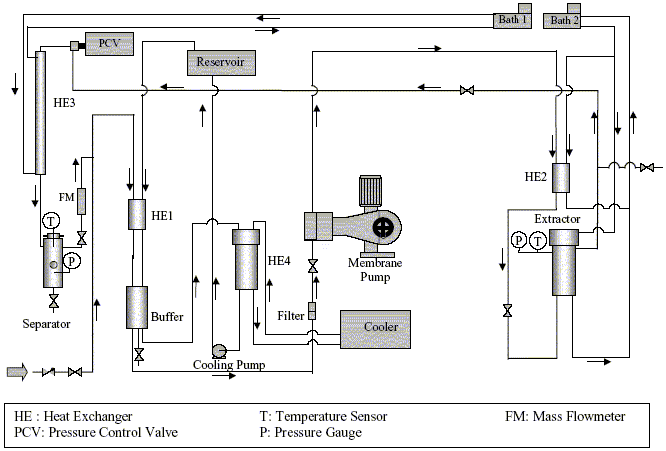 | Figure 1. SC-CO2 Pilot Plant schematic flow |
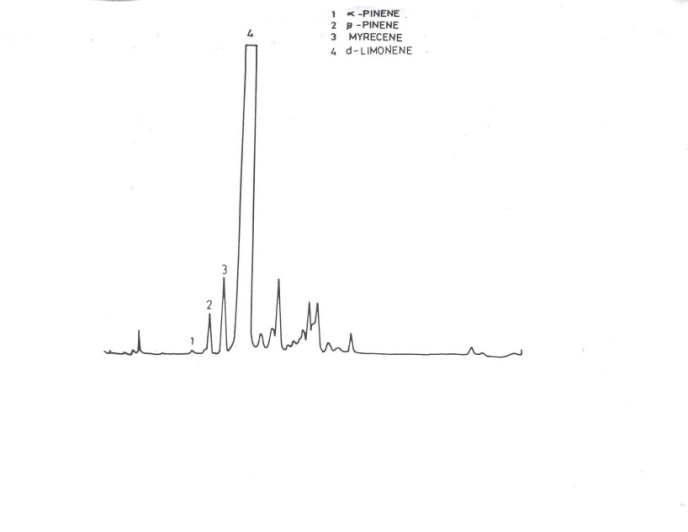 | Figure 2. Orange peel oil analyses by gas chromatography |
2.2. Analysis of Orange Oil
- Analysis of Orange oil (figure 2) was done by using gas chromatography (Perkin-Elmer 8500), column specification and temperature programme are described as follows: column SE30 (10%) on chromosorb W, column material S.S, column length 4 meter, internal diameter 1/8 inch, injector temperature 300℃, FID temperature 300℃, flow rate of N2 30ml/minute and temperature programming 100-250℃ with 5℃/minute rise of temperature, final hold time 5 minute.[20-21]
3. Results and Discussion
3.1. Effect of Pressure on the Extraction of Orange Oil
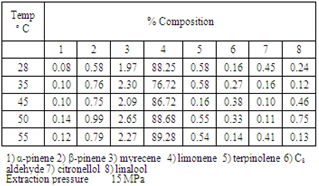
- In this part of study pressure was varied in between 8-25MPa, at 55℃, 5kg h-1 flow rate of SC-CO2 and 2 hour batch extraction time. These data are presented in table 3. With an increase in pressure at constant temperature the yield of the oil was found to increase till 150 bars. Above 150 bars decrease in the degree of extraction was noticed specifically at 20 and 25MPa. Value addition components get extracted in the pressures range of 8-15 MPa. This pressure (table 1) onwards no increase in the yield of the oil was found. Anomalous behavior[18-19] of decrease in yield at high pressures at 55℃ may be due to the obstruction for the flow of SC-CO2 because of decrease in void space of the packed bed. Figure 3 may be referred.[20-21]
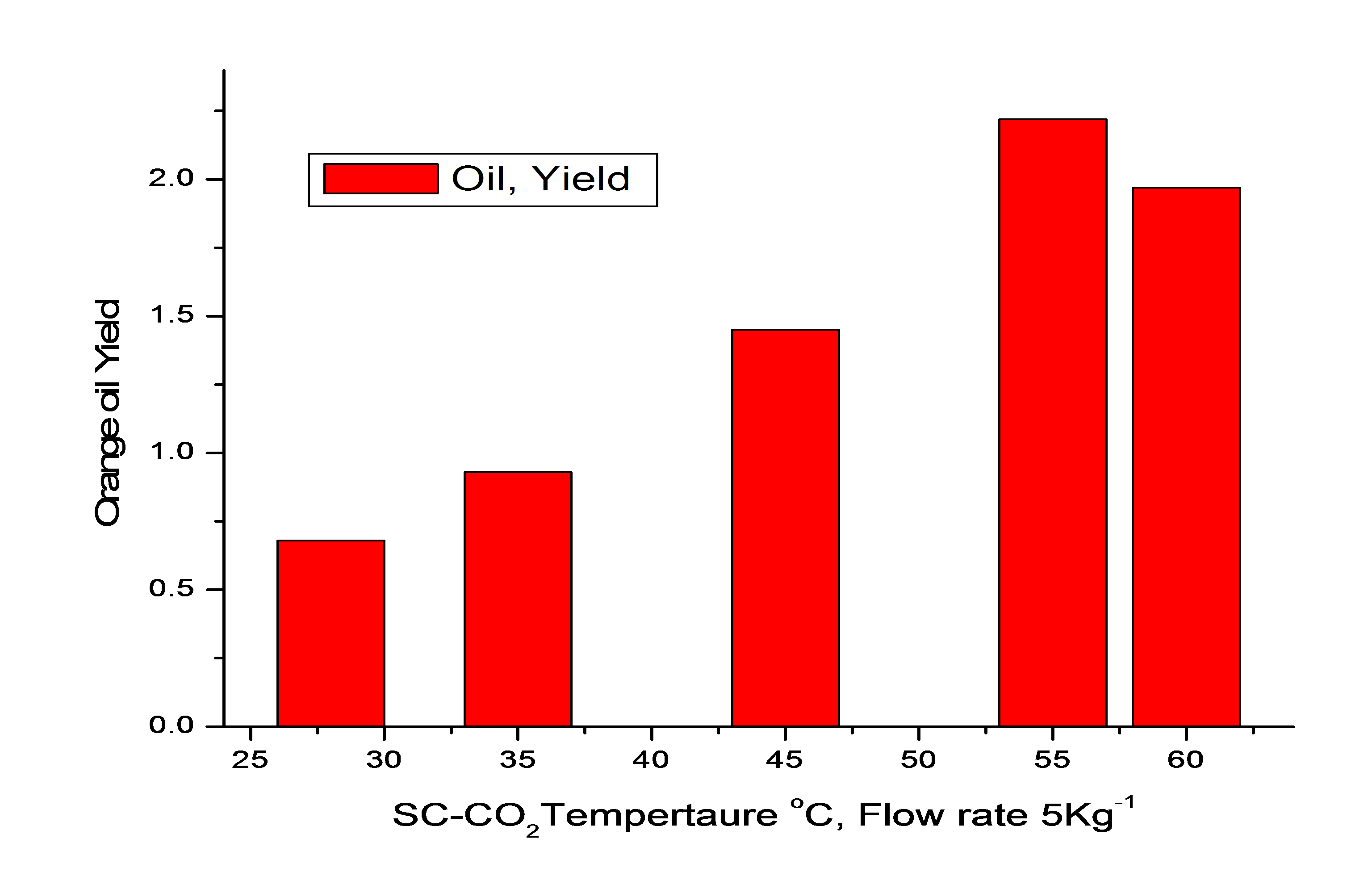 | Figure 3. Effect of temperature on extraction of orange peel oil |
3.2. Effect of Temperature on the Extraction of Orange Oil
- In this part of study, (figure 4) temperature was varied between 28o (subcritical) and 60℃, the pressure was 15MPa, batch time was 2 hour and flow rate of the SFE was 5 kg h-1. It was found that at constant pressure with increase in temperature the yield of orange oil was found to increase till 55℃. At 60℃ the extraction of orange oil (table 2) was found to decrease slightly. Major constituents of the oil, d-limonene was found to increase up to 89.28% at 60℃. The components α-pinene, β-pinene, terpinolene, C8-aldehyde, linalool and citronellol were found to be less than 1% while myrecene was less than 3% in the extracted oil. Thus the temperature of extraction may be kept at 55℃ at 15 MPa pressure, 2 h batch time, and 5kg h-1 flow rate of SC-CO2.[20-21]
 | Figure 4. Effect of pressure on extraction of orange peel oil |
|
|
|
|
4. Conclusions
- The optimum conditions for the volatile oil extraction of orange peels using SC-CO2 were 55℃, 15MPa pressure, 5kg h-1 of flow rate and 2 hour batch time. The yield of the oil was 2.22 wt%. This was certainly highest yield obtained by SC-CO2 against the values reported in the extensive literature search. Quality of oil was absolutely fragrant and resembling as that of fresh orange peel. The color of the oil was light pale yellow color. Various treatments were given to orange peels. The best results were obtained when sun dried ground peels stored in cooling chambers at 0℃ were extracted by hydro distillation. The yield of the oil varied from 3.42 wt%-3.99 wt%. The pH of water was found to be acidic. The oil obtained by hydro distillation was colorless and with more terpenic note. The oil extracted by the SC-CO2 was with very less terpenic.
ACKNOWLEDGEMENTS
- All the Technical assistant staff especially Mr. U.R. Paralkar of Institute of Chemical Technology, Mumbai, deserves special thanks for his efforts in analyzing the oil by GC and in time repairing of the supercritical carbon dioxide pilot plant.
Supporting Information
- Oranges as whole are abundantly used by the food industries in India for recovering the juices. As a result huge amount of peels accumulates over the periods of processing. This could be great concern for the environment due to their biodegradability and hence our efforts were to develop strategically extraction process using SFE technology which itself is a green technology for recovering the Orange oil which has great demands in the international market for the usages in the field of perfume, flavors, pharmaceuticals, beverages, baking industries. It was found to be a successful project and may be exploited on the large scales.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML