-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Obstetrics and Gynecology
p-ISSN: 2326-120X e-ISSN: 2326-1218
2018; 6(2): 32-36
doi:10.5923/j.rog.20180602.02

Genetic Analysis for Prenatal Diagnosis via Amniocentesis at Vietnam National Hospital of Obstetrics and Gyneacology from 2012 to 2016
Cuong Danh Tran1, Van Bich Nguyen1, Minh Xuan Thi Bui2, Lan Ngoc Thi Hoang1, Anh Toan Ngo2, Toan Van Ngo1
1Department of Obstetrics and Gyneacology, Hanoi Medical University, Hanoi, Vietnam
2Center for Prenatal Diagnosis, National hospital of Obstetrics and Gyneacology, Hanoi, Vietnam
Correspondence to: Anh Toan Ngo, Center for Prenatal Diagnosis, National hospital of Obstetrics and Gyneacology, Hanoi, Vietnam.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study aimed at genetic analysis of amniocentesis (karyotypes) for prenatal diagnosis. Methodology: this is a retrospective study among pregnant women indicated for amniocentesis and consent to the study. 11,463 cases were included to the study during 5 years’ period from 2012 to 2016. Results: The rate of successful karyotyping was 99.9%, 10 cases (0.1%) were failure to obtain karyotype. The complication of amniocentesis was miscarriage (0.05%). Acceptance rates for amniocentesis in total number of deliveries were 10.7%, and indications for amniocentesis were mainly high-risk pregnancy such as advanced maternal age, increased nuchal transluciency and abnormalities were observed by ultrasonography. Chromosomal abnormalities are 6.7%, popular conditions were found including trisomy 21(40.6%), trisomy 18 (13.8%), trisomy 13 (2.1%), 45, XO (1.9%). Conclusion: Obtaining fetal fraction by amniocentesis is completely feasible, has great efficacy, safety and helps making decision on the fate of pregnancy.
Keywords: Amniotic fluid, Chromosomes, Amniocentesis, Abnormal fetal chromosome
Cite this paper: Cuong Danh Tran, Van Bich Nguyen, Minh Xuan Thi Bui, Lan Ngoc Thi Hoang, Anh Toan Ngo, Toan Van Ngo, Genetic Analysis for Prenatal Diagnosis via Amniocentesis at Vietnam National Hospital of Obstetrics and Gyneacology from 2012 to 2016, Research in Obstetrics and Gynecology, Vol. 6 No. 2, 2018, pp. 32-36. doi: 10.5923/j.rog.20180602.02.
1. Introduction
- Amniocentesis is considered an invasive procedure for prenatal diagnosis that began hundreds of years ago, which was initially prescribed to drain fluid in the treatment of polyhydramnios. Until 1966, the successful establishment of the fetal chromosome map by amniotic fluid culture has been reported and structural rearrangement of fetal chromosome was diagnosed a year later [1]. In 1968, Valenti and colleagues diagnosed aneuploidy of trisomy 21 in 1968 via amniotic fluid culture [2]. Since then, the method of obtaining fetal specimens by amniocentesis in prenatal diagnosis has been globally applied [3-6]. This study aimed at incidence and classifications of chromosomal disorders among high-risk population that were defined as advanced maternal age, history of malformation birth, abnormal karyotype babies, positive maternal screening tests and etc in Vietnam National Hospital of Obstetrics and Gynecology. This research was conducted in Vietnam from 2012 to 2016 by looking at classical method of karyotyping by amniocentesis.
2. Method
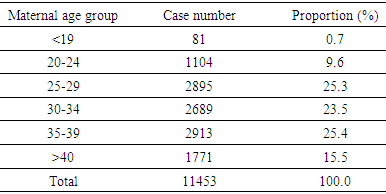
- Study population, sampling and data collection This is a clinic-based, restrospective study. Participants were recruited at Center for Prenatal Diagnosis, National hospital of Obstetrics and Gyneacology, Hanoi, Vietnam which provided full medical records including patients’ medical history, ultrasonographic findings, genetic analysis, consultations. Patients who referred to Center for Prenatal Diagnosis Center are almost all high-risk pregnancy. High-risk pregnancy is defined as following: (1) advanced maternal age, (2) positive maternal serum screening tests at first and second trimester (double test, triple test), (3) previous pregnancies with abnormalities, (4) parents with abnormal karyotype, (5) abnormal ultrasonographic findings. Before performing procedure, amniocentesis is explained by physician and then the patient must give informed consent by signing a form after reading it through. No special precautions are necessary before the start of the procedure, but any medical or other allergies must be communicated to the doctor beforehand, as well as details of all current medications (prescribed or otherwise) and supplements. The urinary bladder should be full in most cases to help elevate the uterus and increase the success rate of the procedure. Once the patient is prepared for the procedure, the vital signs are checked, and an ultrasound examination done to confirm fetal heart rate, placental position, fetal positioning and umbilical cord location. It is also helpful in locating an amniotic fluid pocket that is not occupied by any fetal part, reducing the risk of fetal injury. After cleansing the surface of the abdomen, local anesthetic may be injected followed by the introduction of the ultrasound-guided amniocentesis needle through the anterior abdominal wall, the uterus and the amniotic sac. The uterus may cramp a little at this point. About an ounce of amniotic fluid is extracted using a syringe attached to the needle, and this is sent for testing. After monitoring is completed for an hour, the patient is usually sent home. All patients who agreed to undergo amniocentesis only from January 2012 to December 2016, were recruited to study. The total of 11,463 consecutive pregnant women were taken to the study.Variables, data analysisTotal deliveries were recorded annually in order to emphasize on proportion of amniocentesis and chromosomal abnormalitiy. Indications for amniocentesis were (1) history of chromosomal disorder birth, (2) history of malformation birth, (3) recurrent abortion and miscarriage (≥2 times), (4) parents with abnormal karyotype, (5) positive maternal serum screening test (cut-off of 1/250), (6) advanced maternal age (≥35-year-old), (7) increased nuchal transluciency (≥3mm), (8) abnormal ultrasonographic findings. Statified analyses by gestation age groups (<16-week, 17-week to 20-week, >20-week) are shown in table. Cytogenetic findings were analysed and classified into following categories: (1) aneuploidy of autosomal chromosomes, (2) sex chromosome aneuploidy, (3) structural rearrangements. Patients were consulted about clinical consequences of these findings by both obstetricians and geneticists before giving final decision of pregnancies. Procedure-related complications such as miscarriage, bleeding… were noted as well as failure to harvest adequate cells after culturing. After receiving amniotic fluid, standard G-banding technique was applied to analysed the karyotypes. All data were collected, entered and analysed using SPSS 20.0. Categorical statistics are sumarised in frequency distribution tables. Ethical approval This study was approved by the Ethical Committee of National Hospital of Obstetrics and Gynecology. Participants were informed of the study purpose and were asked to give a written informed consent to confirm their participation. Participants could withdraw anytime and their information was kept confidentially.
3. Results
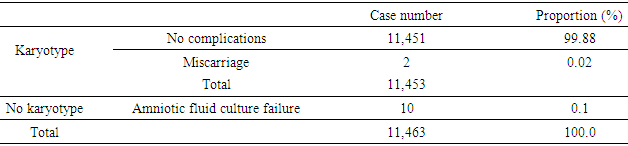
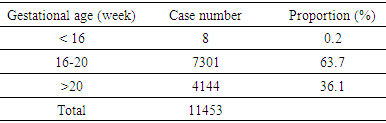
- From 2012 to 2016, 11,463 amniocenteses were performed and analysed at Center for Prenatal Diagnosis, National Hospital of Obstetrics and Gyneacology (Vietnam) however 10 cases were not able to analysed due to culture failure so that total number of karyotype were 11,453 (Table 1).
|
|
|
|
|
|
4. Discussion
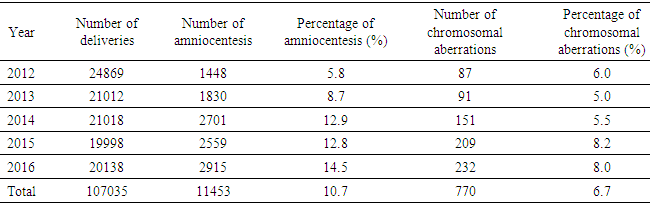
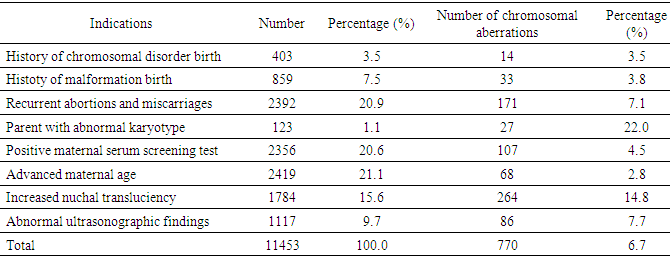
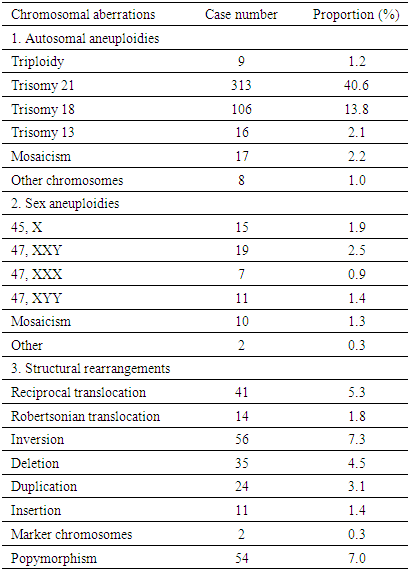
- Amniocentesis is a method of collecting specimens directly from the fetus. This is an invasive procedure that can be used to evaluate fetal genetic conditions especially fetal karyotyping in order to make decision about pregnancy management. Other methods could be used such as chorionic villi sampling and cordocentesis however these methods were not utilized widely compared to amniocentesis in term of procedure-related risk. Amniocentesis rates are as high as 93.0%, 5.0% for CVS and 1.0% for cordocentesis [7]. In another study in Austria, the percentage of amniocentesis was 82.44% and CVS was 17.56% [8]. About achievement of karyotyping after culturing, there were only 10 cases of culture failure due to late pregnancy amniocentesis. Studies has shown the same reason of amniotic fluid culture failure [9]. Statistics significant difference was not expected due to small sample size in term of not-achieving karyotype. Chromosomal abnormalities and indications of amniocentesisIn this study, the rate of chromosomal abnormality was highest in 2015 with 8.2% and the average was recorded at 6.7% (Fig. 1). This proportion was higher than other studies worldwide [4-7]. This can be explained that Center for Prenatal Diagnosis at Vietnam National hospital of Obstetrics and Gynecology is the top-referral hospital in the North of Vietnam which receiving all high-risk pregnancies from Northern provinces. According to present law of Ministry of Health, lower level hospital at provinces are not allowed to perform amniocentesis, thus, it is a burden for the hospital in providing the service to huge number of patients. Parental carriers of chromosomal abnormalities accounted for 1.1% of amniocentesis however 22.0% among those was detected with chromosomal abnormalities, followed by a 15.6% of increased nuchal transluciency with 14.8% chromosomal abnormalities, morphological abnormalities accounted for 9.7% with 7.7% chromosomal abnormalities, positive maternal serum screening tests accounted for 20.6% with 4.5% of chromosomal abnormalities. This finding suggests that the maternal obstetriccondition exploitation plays an important role, especially the abnormal ultrasound-related indications are considered hints of abnormalities. Any abnormalities observed by ultrasonography are indicated for amniocentesis, especially increased nuchal transluciencyat the first quarter. Studies have shown similar results, i.e. ultrasonography is very valuable for detecting fetal malformations to decide on taking amniocentesis. Morrover, ultrasonography could detect 10.0% chromosomal abnormalities [10]. Advanced maternal age was also indicated for amniocentesis, accounting for 21.1% in all cases and among them chromosomal abnormalities was 2.8%. The indications for amniocentesis are similar to those in the world and the rates of chromosomal abnormalities by the indications are similar. At the Center it appeared that all fetal abnormalities, whether alone or in combination with other, were counseled to take invasive procedure [4-6]. Classic cytogenetic technique can only identify numerical chromosomal abnormalities and some kinds of structural chromosomal abnormality in large area of chromatin arms. Specifically, in our study, among all abnormalities, trisomy 21 was the most common (313/770, 40.6%), followed by trisomy 18 (106/770, 13.8%), then trisomy 13 (16/770, 2.1%). Sex chromosomal abnormalities 8.3% in which 45, XO 15/770 (1.9%). The abnormalities of chromosomes 13, 18, and X are reduced because the fetuses carry these aberrations suffering from severe morphological malformations as well as fetal developmental retardation. Therefore, almost all of the woman who received diagnosis expected to terminate pregnancy and refused to undergo amniocentesis. This is reasonable because the indication for fetal karyotyping is only in the fetus abnormalities, but trisomy 18, 13 and 45, XO always accompanied by multiple deformities. According to a study in France, Down syndrome remains the most common chromosome abnormality of about 38% detected by ultrasound, 32% by maternal age and 28% by positive maternal serum screening tests. This study also highlights the role of ultrasound in the diagnosis of Down syndrome, a large part of which is diagnosed after abnormal ultrasonography [7]. Almost all Edward syndrome fetuses have been detected through abnormal ultrasound images, especially in advanced age mother. Similarly, abnormal ultrasound images in combination with advance maternal age can detect 90% of trisomy 18 and 13. One of the most important indications is abnormal morphology in the fetal ultrasonography.The majority of structural rearrangements were inversion (7.3%), polymorphism (7.0%), reciprocal translocation (5.3%), deletion (4.5%). Types of these abnormalities can be observed at Table 6. Chromosomal structure abnormalities, in general, are rare in prenatal diagnosis [11, 12]. Data in previous studies has shown that the rate of visible structure abnormalities was about 0.3 to 2/10,000 live birth [13, 14]. In our health care settings, abortion is permitted while detecting any significant genetic disorder prenatally so that almost all mothers choose to terminate their pregnancies after counselling. That is the reason why chromosomal structural disorders, rarely found in live birth, could only be detected in prenatal diagnosis. Complications Fetal loss is the most common concern for both physician and pregnant women while undergoing amniocentesis. Studies have shown that the incidence of complications around 1% of amniocentesis [7, 8, 15, 16]. In a comprehensive meta-analysis conducted in 2015, the incidence of miscarriage with amniocentesis was 0.11% and with 0.22% for CVS [17]. The risk of this procedure is gradually decreasing due to the improvement of equipment as well as clinical skills. In this study, the overall complication rate was 0.02% (2 cases of miscarriage), so this is a relatively safe method (Table 1). Nonetheless, genetic counseling and risk consideration cannot be ignored before amniocentesis.
5. Conclusions
- Amniocentesis is a very effective way of management of the fetus. It is a simple, easy-to-follow, minimally invasive method according to our study. It not only supplies fetal specimens to the hereditary cells but also provides for other molecular genetic techniques, increasing the probability of detecting fetal disease, thereby, reducing social and familial financial burden in particularly and enhanced population quality in general.
ACKNOWLEDGMENTS
- We would like to thank the participants for their cooperation and the medical staffs from the Center for Prenatal Diagnosis, Vietnam National Hospital of Obstetrics and Gynecology who gave valuable support to this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML