-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Obstetrics and Gynecology
p-ISSN: 2326-120X e-ISSN: 2326-1218
2018; 6(2): 23-31
doi:10.5923/j.rog.20180602.01

Copeptin in Pregnancy Induced Hypertension and Pre-eclamptic Egyptian Women: Relation with CD62p; A Case Control Study
Ola Sayed Mohamed 1, Noha Abdel-Rahman Eldesoky 1, Naglaa Feisal Younis 1, Ahmed Abdel-Aziz El-Mandoury 2
1Biochemistry Department, Faculty of Pharmacy (girls), Al-Azhar University, Egypt
2Obstetrics and Gynecology Department, Faculty of Medicine, 6 Oct University, Egypt
Correspondence to: Noha Abdel-Rahman Eldesoky , Biochemistry Department, Faculty of Pharmacy (girls), Al-Azhar University, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Copeptin, a protein co-secreted with vasopressin, was found to be linked with various diseases; however, its role in pregnancy induced Hypertension (PIH) and pre-eclampsia (PE) is still unclear and up to our knowledge, this is the first time to investigate its concomitant usage together with CD62P, an established marker of platelet activity, for the early diagnosis of PE. Objective: To investigate serum copeptin level in PIH and PE and to study the efficacy of dual usage of serum copeptin and CD62P in early diagnosis of PE. Methods: This study was conducted on seventy five females in the third pregnancy trimester divided into three groups: 15 normal pregnant women, 30 pregnant women with PIH and 30 with PE. Serum copeptin and CD62P were measured by ELISA. Complete blood picture, Platelet count, prothrthrombin time and prothrombin concentration were measured and albumin in urine was detected by dip stick method. Results: serum levels of copeptin and CD62P were significantly higher in PE group compared to PIH and control groups at (p≤0.01). The diagnostic performance of copeptin serum level showed specificity and sensitivity of 100% and 73.3% respectively. Moreover, dual assessment of serum copeptin and CD62P together revealed enhanced specificity and sensitivity of 100% and 96.7% respectively for discriminating PE from PIH patients. Conclusion: Serum copeptin level could be used as an important biomarker for the early diagnosis of pre-eclampia. Moreover, the involvement of copeptin and CD62P in the diagnosis of PE provides a more valuable diagnostic tool than single-marker measurement.
Keywords: Pregnancy Induced Hypertension, Pre-eclampsia, Copeptin, CD62p, p-selectin, Platelet Activation
Cite this paper: Ola Sayed Mohamed , Noha Abdel-Rahman Eldesoky , Naglaa Feisal Younis , Ahmed Abdel-Aziz El-Mandoury , Copeptin in Pregnancy Induced Hypertension and Pre-eclamptic Egyptian Women: Relation with CD62p; A Case Control Study, Research in Obstetrics and Gynecology, Vol. 6 No. 2, 2018, pp. 23-31. doi: 10.5923/j.rog.20180602.01.
Article Outline
1. Introduction
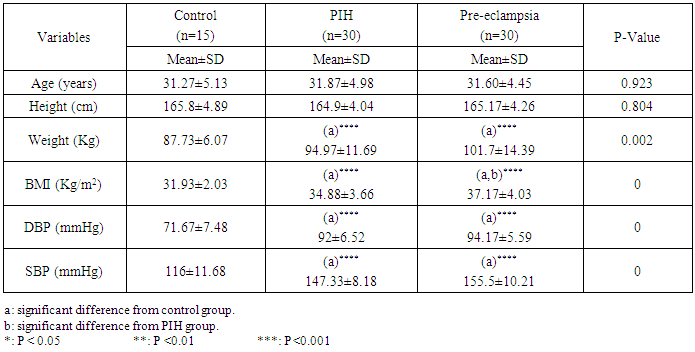
- Hypertension is the most common medical problem encountered during pregnancy and remains an important cause of maternal, and fetal, morbidity and mortality. It complicates up to 2-3 % of pregnancies [1, 2]. According to the World Health Organization, hypertensive disorders account for 16% of all maternal deaths in developed countries, its incidence is seven times higher in developing countries (2.8% of live births) than in developed countries (0.4%) [3].Pregnancy induced hypertension is defined as hypertension (blood pressure ≥ 140/90 millimeters of mercury) without proteinuria and diagnosed after 20 weeks gestation, while pre-eclampsia is defined as new onset proteinuria (≥ 300 mg/24 hours) in hypertensive women who exhibit no proteinuria before 20 weeks gestation [4]. The lack of mechanistic understanding makes early diagnosis and prevention of pre-eclampsia nearly impossible. Therefore, the identification of new biomarkers for early diagnosis is of great importance. Moreover, novel biomarkers may lead to the identification of new therapeutic targets that could result in the development of more effective treatment options for this devastating disease. In the present work, we focused on serum Copeptin level and one of platelet activity markers; CD62p, in PIH and PE Egyptian women.In pre-eclampsia, food supply to the placenta is inadequate, due to improper vascularisation of the placenta [5], followed by placental ischemia creating a hypoxic environment which favors oxidative stress, consequent oxidative damage and inflammation [6].If the placenta is not functioning properly, the extra blood supply that should be pushing through from the mother will not go through, causing hypertension, swelling and possibly kidney problems. Mother cannot eliminate waste products fast enough so they build up in her blood, while certain vital proteins that should stay in the bloodstream leak into her urine, causing proteinuria [7].It is well known that normal pregnancy is characterized by an increase in platelet aggregation and a decrease in the number of circulating platelets. Increased consumption of platelets in the uteroplacental circulation has been suggested to be an explanation of the reduction in the number of circulating platelets [8].In pre-eclampsia, the generalized endothelial alteration causes vasoconstriction and promotes adhesion and aggregation of platelets, as well as the activation of the coagulation factors, inducing supplementary hypoxic injury of the endothelium [9].It has been reported that arginine vasopressin (AVP) may be involved in the pathophysiology of pre-eclampsia. However, its short half-life and instability makes its measurement nearly impossible [10].Copeptin is a peptide containing 39 amino acids. It is cosecreted in an equimolar amount with AVP from the neurohypophysis upon hyper osmotic stimuli. It is believed to be involved in the proper folding of pre-pro-AVP. Although AVP’s function is regulation of blood volume and electrolyte homeostasis, copeptin’s function is still not fully understood [11].Unlike AVP, copeptin is stable both in serum and plasma at room temperature and can be easily measured as a surrogate for AVP [12]. Recently, copeptin has been considered as a promising biomarker in various acute diseases as sepsis, stroke, and acute pancreatitis [13]. However its use as a biomarker for PE is still under study.CD62p (P-selectin) is a protein stored in platelets alpha-granules and endothelial cells Weibel-Palade bodies. CD62p functions as a cell adhesion molecule (CAM) on the surfaces of activated endothelial cells, which line the inner surface of blood vessels, and activated platelets [14]. It plays an essential role in the initial recruitment of leukocytes to the site of injury during inflammation. When endothelial cells are activated by molecules such as histamine or thrombin, CD62p moves from an internal cell location to the endothelial cell surface. Recent studies suggest an additional Ca2+ independent pathway involved in CD62p release [15].
2. Materials and Methods
2.1. Study Population
- This study is a case control study that was conducted on 75 pregnant women. The study populations were selected from pregnant women in the third trimester of pregnancy who attended Kasr Al Ainy Hospital in the period from March 2016 to August 2016. All participants were examined clinically before sample collection and categorized into three groups; 15 normal pregnant women (free from any medical disorders), 30 PIH women with blood pressure >140/90 mm Hg, 30 PE women with blood pressure >140/90 mm Hg and proteinuria.The study protocol was approved by the hospital ethical committee and the Helsinki Declaration of 1975. An informed consent was taken from the patients prior to their enrolment in this study. Patients with history of renal, hepatic, cardiac disease or any metabolic disorder that may affect the studied biochemical parameters were excluded.
2.2. Blood Samples
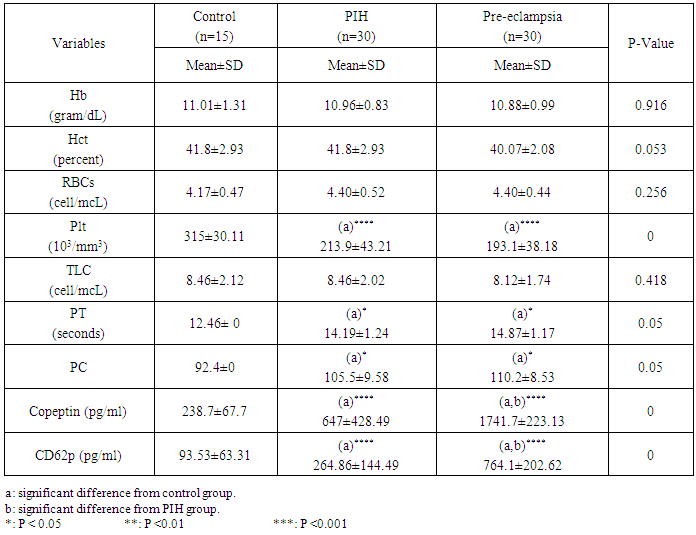
- Blood samples were collected according to the recommendations of national committee of clinical laboratory standards. Seven ml of venous blood was collected from each subject after overnight fasting by vein puncture. Each blood sample was divided into three portions: Two ml were collected into (K3 EDTA) containing tubes for complete blood picture including haemoglobin, haematocrit, RBCs Count, Platelet Count and Total leucocytic Count by using fully automated hematology cell counter SYSMEX XT-2000i which works on the principle of light deflection method [16], Two ml were collected into citrate tubes then centrifuged to separate plasma for determination of prothrombin time and prothrombin concentration by using automated coatron A4 [17], Three ml were collected in vaccutainers, left to coagulate adequately before centrifugation at 3000 rpm for 10 minutes followed by serum separation. Serum obtained was divided into 2 aliquots and stored at -40ºC until the time of copeptin and CD62p assay. Serum Copeptin was determined by an ELISA technique using a Copeptin kit obtained from WKEA med supplies (China) [18].Serum CD62p was determined by an ELISA technique using a CD62p kit obtained from WKEA med supplies (China) [19].
2.3. Urine Samples
- Urine samples were collected in clean, sterile containers, mixed well then protein in urine was detected using dipstick method and the color obtained was compared with a chromatic scale. The color was progressed through various shades of green to blue. Reading was reported in terms of negative, trace, 1+, 2+, 3+ and 4+ corresponding to each change. Pre-eclampsia is diagnosed with BP of >140/90 mmHg, in combination with proteinuria (300 mg/24 h or ≥1+ by dipstick) on at least 2 occasions measured at least 4 h apart. It is classified as mild by the presence of proteinuria of 1+, without evidence of end-organ damage and as severe by the presence of proteinuria of more than 5 g in 24 h, or ≥3+ on 2 random urine samples [20].
2.4. Statistical Data Analysis
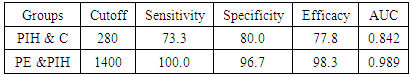
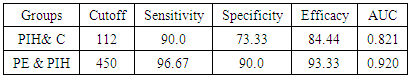
- IBM SPSS statistics (V. 24.0, IBM Corp., USA, 2016) was used for data analysis. Data was expressed as Mean±SD for quantitative parametric measures in addition to both number and percentage for categorized data.The following tests were done:1. Comparison between the 3 groups for parametric data using Analysis of Variance (ANOVA).2. Pearson correlation test to study the possible association between each two variables among each group for parametric data.3. Diagnostic validity test: the receiver operating characteristics (ROC) curve was constructed to obtain the most sensitive and specific cutoff for both copeptin and CD62p. To evaluate the most discriminating marker between the compared groups, area under the curve (AUC) also has been calculated.
3. Results
|
|
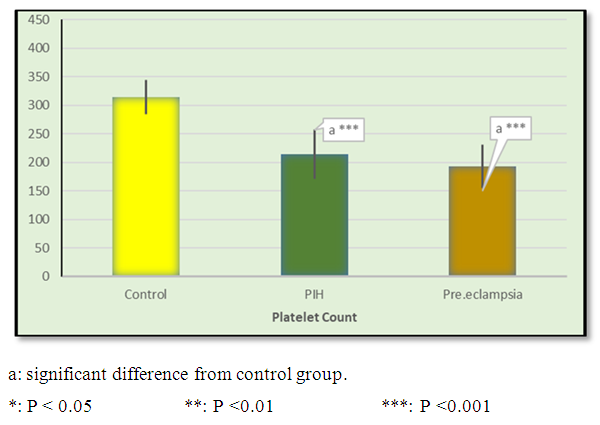 | Figure 1. Mean ± SD of platelet count in control, PIH and pre-eclamptic groups |
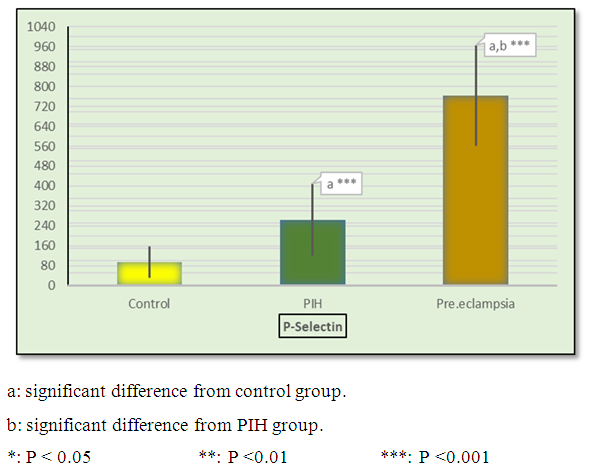 | Figure 2. Mean ± SD in CD62p in control, PIH and pre-eclamptic groups |
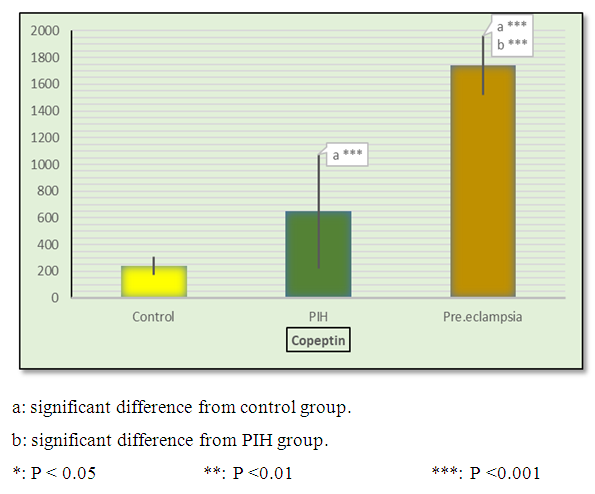 | Figure 3. Mean ± SD of copeptin in control, PIH and pre-eclamptic groups |
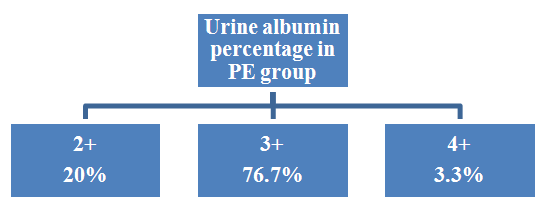 | Figure 4. Urine albumin percentage in PE group |
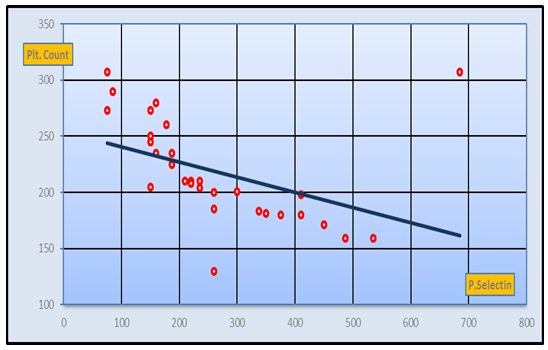 | Figure 5. Negative Correlation between CD62p and platelet count in PIH group (r-value= -0.54 at p-value= 0) |
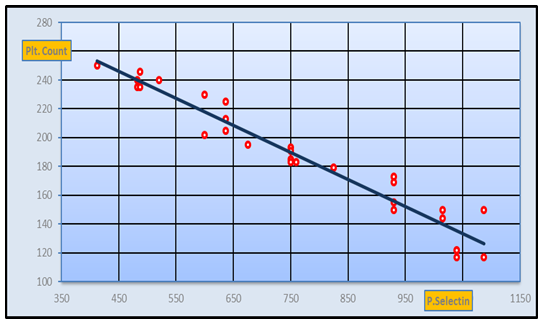 | Figure 6. Negative Correlation between CD62p and platelet count in pre-eclamptic group (r-value= -0.86 at p-value=0) |
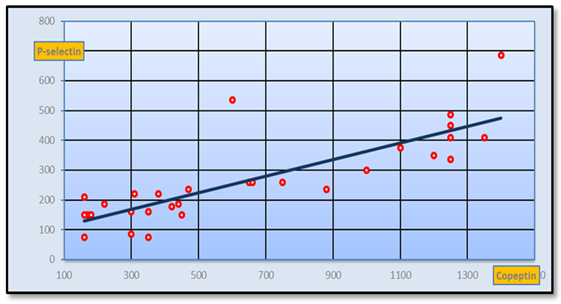 | Figure 7. Positive Correlation between CD62p and copeptinin PIH group (r-value=0.821 at p-value=0.01) |
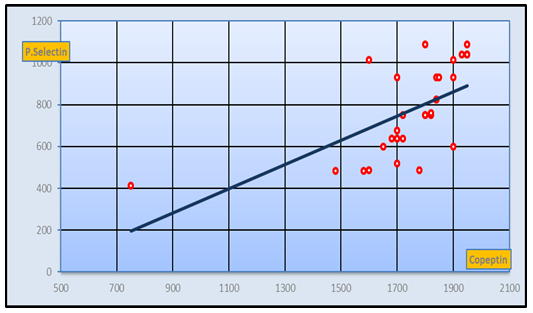 | Figure 8. Positive Correlation between CD62p and copeptin in pre-eclamptic group (r-value= 0.73 at p< 0.05) |
|
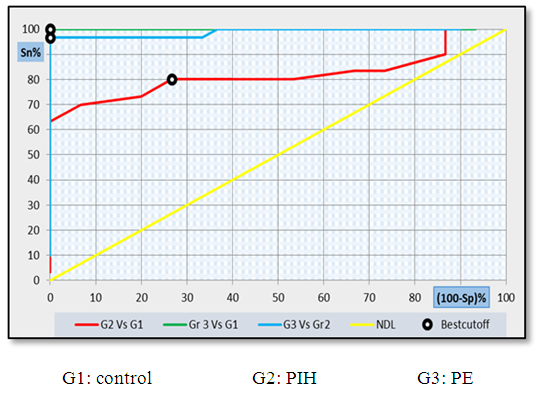 | Figure 9. ROC curve analysis showing the diagnostic performance of Copeptin for discriminating different groups from each other's |
|
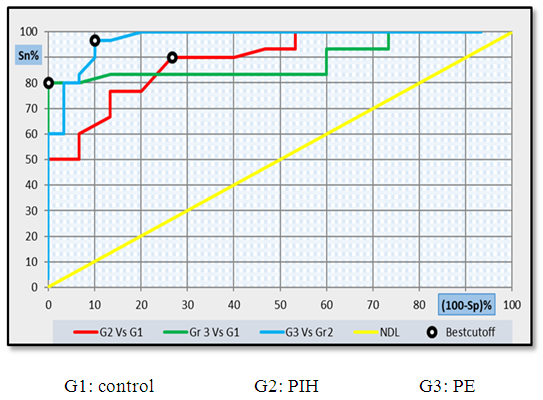 | Figure 10. ROC curve analysis showing the diagnostic performance of CD62p for discriminating different groups from each other's |
|
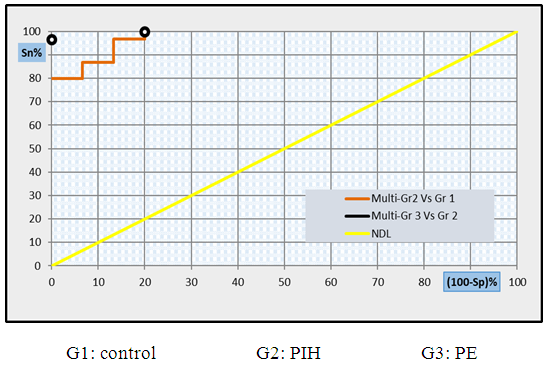 | Figure 11. Multi- ROC curve analysis showing the diagnostic performance of CD62p and copeptin for discriminating different groups from each other's |
4. Discussion
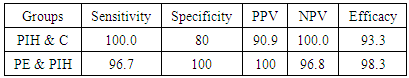
- Hypertension, a serious pregnancy complication, and its severe forms (pre-eclampsia and eclampsia), are characterized by abnormalities in clotting and a great spread damage to the endothelium resulting in vasoconstriction and promotion of platelet aggregation and adhesion with further hypoxic damage to the endothelium [21].In the current study we have focused on the potential use of copeptin and CD62p as predictive markers of PE.In the present study age range included almost all the women′s sexally active interval (20 to 40 years) in all studied groups, Weight and body mass index were significantly higher in PIH and PE groups compared with control group (Table 1).These results were in accordance with the findings reported by Ramsey et al., Yeung et al., Alkinade et al. and Bartsch et al. who reported that women with pre-eclampsia were significantly more likely to have higher body mass index and deliver lower birth weight babies [22, 23, 20, 24]. Also Fan et al. found pregnancy maternal obesity an important risk factor for the development of PE [25].These results were disagreed with Zulfikaroglu et al. who didn′t find any statistical difference between the mean ages, BMI or gestational ages at sampling of PE group and normal pregnant women [26].In the present study systolic and diastolic blood pressures were significantly higher in PIH, PE groups compared to control group (Table 1).Stepan et al. defined PIH as (blood pressure ≥140/90 mmHg) without proteinuria and diagnosed after 20 weeks gestation, while pre-eclampsia is a new onset proteinuria (≥ 300 mg/24 hours) in hypertensive women after 20 weeks gestation [27].In the present work, there were no significant differences in Hb, HCT, TLC and RBCs between control, PIH and PE groups (Table 2).These results were in accordance with the findings reported by Chandra et al. [28]. However Han et al. stated that a high hematocrit value could be a sign of pre-eclampsia and explained their findings by the theory that PE often causes the body's tissues to absorb blood plasma. The blood becomes more concentrated, resulting in an abnormally high hematocrit value [29].In the present work there was highly significant decrease in Platelet count in PIH and PE groups than control group (Table 2 & Figure 1).These results were agreed with Michael and Braun who stated that PE may cause an abnormally low platelet count (Thrombocytopenia) [30].Juan et al. explained that by consumption of platelets during low grade intravascular coagulation [8]. Platelets also appear to circulate in a more activated state and tend to adhere to the damaged endothelium. While Alkholy et al. explained that by increased platelet destruction and platelet turn over in pre-eclampsia [31].In the present work there was significant increase in PT and PC in PIH and PE groups than control group (Table 2).Adaeze et al. found significant increase in PT and activated partial thromboplastin time (TT) of hypertensive pregnant females when compared with normotensive ones [32].Han et al. reported that normal pregnancy causes a maternal physiological hypercoagulable state in late pregnancy. Pre-eclampsia may trigger complex disorders in the endogenous coagulative pathways and consumes platelets and fibrin, subsequently activating thrombopoiesis and fibrinolysis [33].Shetty et al. stated that significant prolongation of PT in PIH indicates consumption of coagulation factors, especially factor VIII [34].However these findings were disagreed with Chandra et al. who found no marked changes in PT or TT accompaning PIH women [28].CD62p is a thrombo-inflammatory molecule involved in platelet activation and aggregation [35] Platelets are the major source of circulating CD62p. Elevated levels of this protein have been found in many atherothrombotic disorders. Thus CD62p level could be proposed as a reliable marker of platelet activation [36] and also as a marker of inflammation [15, 37]. We have measured CD62p concentration to explore its maternal levels during PIH and PE in the third trimester of pregnancy.The studied groups showed highly significant increase in CD62p concentration in PIH and PE groups compared to control, also there was significant increase in pre-eclamptic group compared to PIH (Table 2 & Figure 2).These findings were in accordance with Vladareanu et al. who indicated that in pre-eclamptic women, the platelets showed more extensive activation, indicated by the increased expression of CD62p [9].Japp et al. stated that surface expression of CD62p on activated platelets induced formation of platelet monocyte aggregates and promoted vascular inflammation and thrombosis, thus CD62p antagonism may represent a novel therapeutic strategy in vascular disease [38].In the present work, there was a significant increase in copeptin concentration in pre-eclamptic group compared to PIH group and a significant increase in PIH group than control group (Table 2, Figure 3).These results were agreed with Santillan et al. who found copeptin significantly higher throughout pre-eclamptic pregnancies versus control pregnancies. They claimed that chronic infusion of AVP during pregnancy (24 ng per hour) is sufficient to develop pre-eclampsia [39]. Also Sandgren et al. stated that AVP secretion is elevated starting from the 6th week of gestation in women who later developed PE [40].Moreover, Zulfikaroglu et al., Cornelius and Yeung et al. found that increased copeptin levels were more strongly associated with severe than mild pre-eclampsia [26, 10, 23].Also Yeung et al. found serum copeptin higher in pregnant women who go on to develop pre-eclampsia. However they indicated that levels of copeptin were not elevated in women with other pregnancy complications, including gestational diabetes mellitus, gestational hypertension and preterm birth [23]. This counteracts our results that involved significant elevation in copeptin level in PIH than control.In the present work, urine albumin was absent in each of PIH and control groups, while in PE it was present and the intensity of colour of test strips gave an indication on the amount of albumin in urine (Figure 4).Explanation of increased urine albumin in pre-eclamptic group was suggested by Cunningham et al., (2013) and Magee et al., (2014) who stated that renal vasospasm in pre-eclampsia leads to decreased renal perfusion, decreased glomerular filtration rate and glomerular lesion leading to leakage of essential proteins into the mother’s urine [41, 42].In the present work there was negative significant correlation between CD62p and platelet count among PIH and PE patients groups (figure 5 & 6).Merten and Thiagarajan found CD62p mediates rolling of platelets and leukocytes on activated endothelial cells. After platelet activation, CD62p is translocated from intracellular granules to the external membrane, whereas fibrinogen aggregates platelets by bridging glycoproteinIIb/IIa between adjacent platelets and expression of CD62p on the platelet surface correlates strongly with the mean platelet aggregate size [43].Also Holmes et al. found positive correlation between CD62p and platelet count in pregnant woman at 20 and 35 weeks [44], and Trugilho & his colleagues found the same correlation in patients with thrombocytopenia [45].In the present work there was positive significant correlation between CD62p and copeptin among PIH and PE groups (figures 7, 8). This could be explained by the physiopathology of PE that involves altered endothelium function and platelet activation that is linked to selectins [46, 44].The mechanism of platelet aggregation induced by AVP was studied by Colucci et al. in human platelet rich plasma and AVP was found to cause a dose-dependent aggregation with a concomitant stimulation of thromboxane B2 formation [47]. On top of that Zagajewska et al. found that copeptin could also be a biomarker of inflammation [48]. In the meantime, Geng et al. stated that CD62p plays key roles in mediating inflammation through promoting adherence of leukocytes to activated platelets and endothelium [49]. George et al. revealed that transcription of CD62p mRNA is induced by inflammatory mediators such as tumor necrosis factorα (TNFα) and interleukin 4 (IL4) reactions, this could explain the positive significant correlation between CD62p and copeptin [37].In the present study ROC curves were carried out to assess the diagnostic performance of copeptin and its sensitivity and specificity in control, PIH and pre-eclamptic groups in third trimester (table 3, figure 9).The best cutoff value of copeptin that could discriminate between PIH group & control group was found at 280, below which, the Sp% = 80% and above which, the Sn% =73.3%. The negative predictive value =85.7, positive predictive value =64.7%, while efficacy = 77.8%.The best cutoff value of copeptin that could discriminate between pre-eclamptic group &PIH group was found at 1400, below which, the Sp% =96.7% and above which, the Sn% =100%. The predictive negative value =100%, predictive positive value =96.8%, while efficacy = 98.3%.Our results are in accordance with Santillan et al. who analyzed their results using ROC curve and found copeptin significantly predictive of the development of pre-eclampsia in all trimesters when controlling for covariates such as age, BMI, chronic hypertension and history of pre-eclampsia [39].In the present study ROC curves were carried out to assess the diagnostic performance of CD62p and their sensitivity and specificity in control, PIH and pre-eclamptic groups in third trimester (table 4, figure 10).The best cutoff value of CD62p that could discriminate between PIH group & control group was found at 112, below which, the Sp% = 73.33% and above which, the Sn% = 90%. The negative Predictive value = 87.57%, positive Predictive value = 87.10, while efficacy = 84.44%.The best cutoff value of CD62p that could discriminate between pre-eclamptic group & PIH group regarding CD62p was found at 450, below which, the Sp% = 90.0% and above which, the Sn% = 96.67%. The negative predictive value = 96.67%, positive predictive value = 90.63%, while efficacy = 93.33%.In the present study multi ROC curve was carried out to assess the diagnostic performance of dual usage of CD62p and copeptin and their sensitivity and specificity in control, PIH and pre-eclamptic groups in third trimester (table 5, figure 11).Muli-ROC of CD62p and copeptin that could discriminate between PIH group & control group has Sp% = 80.0% and Sn% =100%. The negative predictive value = 100%, positive Predictive value = 90.9, while efficacy = 93.3%.Muli-ROC of CD62p and copeptin that could discriminate between pre-eclamptic group &PIH group has Sp% = 100.0% and Sn% =96.7%.The negative predictive value = 96.8%, positive Predictive value = 100%, while efficacy = 98.3%.
5. Conclusions
- Our results imply apotential role of copeptin as a predictive biomarker of PE in high risk group as those suffering from PIH. Moreover, dual assessment of copeptin and CD62p could provide better discrimination of PE patients from PIH patients and could pick the early conversion of PIH into PE than the use of each of them alone.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML