-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Obstetrics and Gynecology
p-ISSN: 2326-120X e-ISSN: 2326-1218
2016; 4(2): 17-26
doi:10.5923/j.rog.20160402.01

Metformin Opposed to Insulin in the Management of Gestational Diabetes
1Obstetrics & Gynecology Department, Faculty of Medicine, Tanta University, Egypt
2Internal Medicine Department, Faculty of Medicine, Tanta University, Egypt
Correspondence to: Hesham Borg, Obstetrics & Gynecology Department, Faculty of Medicine, Tanta University, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Gestational diabetes mellitus (GDM) is linked with amplified risk of a variety of maternal and perinatal complications including preeclampsia, cesarean section, macrosomia, shoulder dystocia, birth injuries, hypoglycemia, and respiratory distress syndrome. Purpose: to assess the effectiveness of Metformin in opposition to insulin for the treatment of GDM. Subject and Methods: This was an experimental prospective comparative study done at Obstetrics and Gynecology Department of Tanta University Hospital, Egypt. One hundred patients were selected having gestational diabetes at 22-36 weeks of gestation with singleton pregnancy to be included in this study. Group A (Metformin group) (n=50) were given Metformin at an oral dose of 500 mg daily in the morning and was increased when necessary by 500 mg weekly up to total of 2500 mg per day, Group B (Insulin group) (n=50) were given insulin at a dose of 0.7 U/kg body weight, given subcutaneously twice daily, and was increased as needed. The patients were subjected to history taking, clinical examination, laboratory investigations as FBS, 2hPPBS, HbAlc before initiation of therapy and after treatment. Standard obstetric care was accessible at the antenatal clinics including ultrasound examination. A neonatal assessment was done by clinical examination (for macrosomia, congenital malformations) and by laboratory investigations for hypoglycemia and Hyperbilirubinemia. Results: The Metformin was found to be as effective as insulin for the control of gestational diabetes as it reduced FBS, 2hPPBS and HbAlc significantly (P <0.001), (P <0.001) and (P <0.001) respectively, Metformin was beneficial in reducing maternal weight gain during pregnancy whereas insulin group had weight gain values statistically higher than Metformin group (P<0.001). Also, Metformin was better than insulin in reducing neonatal hypoglycemia (P=0.039). There was no significant difference between Metformin and insulin as regard mode of delivery (P=0.685), development of gestational hypertension or preeclampsia (P=0.204), neonatal birth weight (P=0.780) and neonatal jaundice (P=0.229). Conclusions: Metformin is effective as insulin for the management of GDM. Metformin can be used securely during pregnancy as it is not linked with congenital malformations or increased maternal or neonatal complications. Metformin decreases the rate of neonatal hypoglycemia. Insulin is still the gold standard for management of gestational diabetes.
Keywords: Gestational, Diabetes, Metformin, Insulin
Cite this paper: Hesham Borg, Sherif Ezat, Metformin Opposed to Insulin in the Management of Gestational Diabetes, Research in Obstetrics and Gynecology, Vol. 4 No. 2, 2016, pp. 17-26. doi: 10.5923/j.rog.20160402.01.
1. Introduction
- Gestational Diabetes has been defined as any level of glucose intolerance with onset or first detected throughout the pregnancy. The morbidity of Gestational Diabetes Mellitus (GDM) is expanding. Depending on the population studied, classification of diabetes and the diagnostic tests in use nearly 1–14% of all pregnancies are complicated by GDM. [1] GDM is linked with numerous unfavorable outcomes in the short- and long-term for both mother and her offspring. [2] GDM for all time accompanies an increased maternal hazard of preeclampsia, cesarean section, in addition to an increased risk for developing type 2 Diabetes (T2D) after pregnancy. [3, 4] As well, there is a greater than before risk for neonatal loss, still birth and congenital defects [5] resulting from excessive mother-to-fetus glucose transfer. [6, 7] A further major complication is macrosomia, which is a risk factor for instrumental delivery, cesarean section and shoulder dystocia during delivery and neonatal hypoglycemia directly after birth [8]. Moreover, the influence of the intrauterine hyperglycemia surroundings might go with the children afterward. [3, 9] So the management of GDM is first and foremost aimed at controlling glycemic level to decrease the occurrence of unpleasant pregnancy outcomes. Crowther CA, et al. (2005) had established that thorough treatment of women with GDM reduced birth weight and incidence of macrosomia in infants born to mothers who had participated in the intervention compared to women who had received routine care. [10] Consequently, active treatments such as dietary therapy, exercise, oral hypoglycemic agents, Insulin are essential to lessen the complications. [11] As proper diet, only or associated with physical exercise, are not sufficient to control blood glucose levels in pregnant women, subcutaneous Insulin therapy has been considered the gold standard for the management of GDM. [12-14] On the other hand, Insulin has several disadvantages including many daily injections, the risk of hypoglycemia and maternal weight gain. [15] It requires adjustment based on the patient's body mass index, glucose levels and the way of life. [16] As a result, detailed guidance for dose change of Insulin is essential to make sure of the safe self-administration of Insulin. Unsurprisingly, safe and effective oral therapy would be more satisfactory still extremely preferred for women with GDM. [2, 17] Though, it is indispensable to understand the effects of oral hypoglycemic agents on both maternal and neonatal outcomes for the women with GDM. For T2D Metformin, is the first line of management, sits in the hopeful list. Given that Metformin has been found to have a maternal-to-fetal transfer rate of 10–16% which might be associated with fetal anomalies, potential adverse effects for mothers and the newborns after delivery, it has not been widely used in GDM. [18, 19] There are, many studies focused on examining the efficiency and safety of Metformin in the management of GDM. Some of these studies are case-control trials [20, 22], or observational studies [23], others are randomized controlled trials (RCTs) but with small samples missing the influence to describe confirmative conclusions on the relative risks and benefits of Metformin for GDM. Also, few studies were done in Egypt, of them a study done by Magdy A. Mohamed et al., Sohag University 2014, and the study done by Hend S. Saleh et al., Zgazig University 2016. [24, 25] So the use of Metformin is still controversial in pregnant women, therefore, the aim of this study was to compare the effects of Metformin and Insulin on glycemic control, maternal and neonatal outcomes in GDM.
2. Subjects and Methods
- This experimental prospective comparative study was carried out in The Department of Obstetrics & Gynecology, Tanta University Hospital through May 2014 to January 2015. One hundred patients were included in this study from pregnant females with gestational diabetes attended antenatal clinics of Tanta University Hospital. The sample size was determined using the following formula (n=2*Cp, power/d2), where n is the number of subjects required in each group, d is the standardized difference and Cp, power is a constant defined by values chosen for the p value and power required (for p value 0.05 & power 90% the constant 10.5). The standardized difference (d) equal Target difference/standard deviation=15/21=0.71.n=2*10.5/(0.71)2 = 41.65. So the least sample size in each group 42 & total sample size 84. For purpose of increasing validity and for any factor of discontinuation of study during follow up 10 % of cases are added to the sample size thus 100 cases were included in this study. The patients were selected having gestational diabetes diagnosed according to the Carpenter and Coustan criteria (fasting > 95 mg / dl, 1 h > 180 mg/ dl, 2 h > 155 mg / dl, and 3 h > 146 mg / dl.). [26] Using 2 or more abnormal plasma glucose values at 22-36 weeks gestation with singleton pregnancy and patients did not respond to diet modifications or nutritional instructions alone in 2 weeks and patients met the Tanta University Hospital criteria for starting insulin therapy. Patients with contraindication to Metformin, fetal anomaly, gestational hypertension, preeclampsia, fetal growth restriction, ruptured membranes, and patients in labor were excluded from this study. Patients were consecutively ordered. Random tables were used to randomize those patients into two equal groups (metformin was categorized as letter “M”, and the patients who used insulin therapy was categorized as letter “I”. Both “M” and “I” tickets were put in opaque envelops according to consecutive orders got from the random tables. After counseling women about the nature of the study and after gaining a written consent, the corresponding consecutive envelop was unlocked and the patient then received the corresponding drug according to the type of ticket inside “M” or “I”. Thus Participant patients were divided into two groups: A) Metformin group: included 50 pregnant females with GDM treated by Metformin (B-Bigunide) at oral dose 500 mg daily in the morning and increased when necessary by 500 mg weekly up to total dose 2500mg/day.B) Insulin group: included50 pregnant females with GDM treated by insulin, (Mixtard); mixed human suspension (‘crystalline 30% + protamine insulin 70%’) will started at dose of 0.7U/kg of body weight given subcutaneous twice daily and will be increased as needed.Patients were subjected to history taking, clinical examination, and the glycemic profile, fasting blood sugar (FBS), and postprandial blood sugar (2 hr PPBS) were done weekly for all cases. Dose alterations of medications were made at each antenatal appointment weekly till delivery. Standard obstetric care was accessible at the antenatal clinics including ultrasound examination which was done at first appointment (dating scan) and then at 16–19 weeks (anomaly scan) and then once-a-month after 28 weeks as fetal well-being scan. HbAlc was done at entrance of study and at around 37 weeks of pregnancy. Method and time of delivery were decided around 38 weeks of pregnancy. Maternal outcome in the form of glycemic control, medical problems, and mode of delivery were recognized. Neonatal assessment was done by clinical examination (for macrosomia, congenital malformations) and by laboratory investigations for hypoglycemia and Hyperbilirubinemia, and all were statistically analyzed.Ethical considerations: Before starting the study, an approval from The Ethical Committee of Faculty of Medicine, Tanta University was obtained. All aspects of this study were completely explained for all the participant patients and a written informed consent was taken from them.Statistical Analysis: using IBM SPSS software package version 20. Dichotomous data were described using number and percent. Continuous data were described using Range (minimum and maximum), mean, standard deviation (SD). Comparison between Metformin and Insulin group regarding categorical variables was tested using Chi-square test. Non parametric tests as Mann Whitney test was used with abnormal distribution data. Comparison between Metformin and Insulin groups were done using independent t-test. Paired t-test is used to analyze two paired data. Significance test results are quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level. P ≤ 0.05 was considered statistically significant.
3. Results
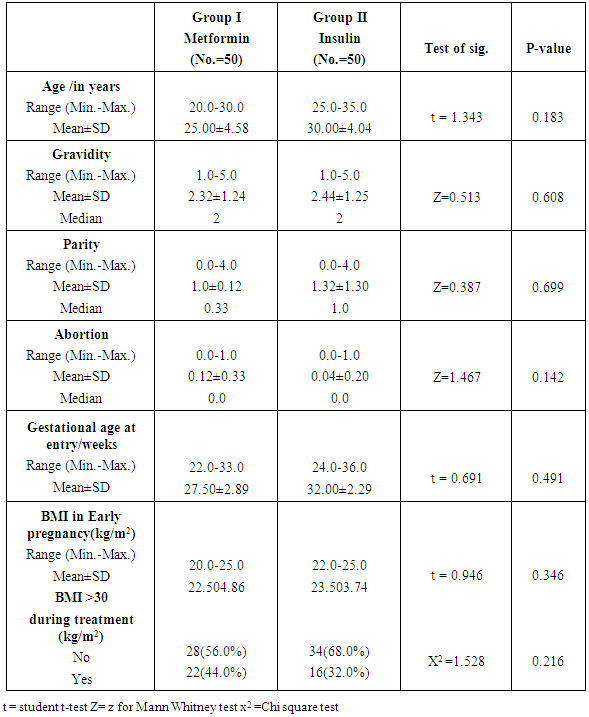
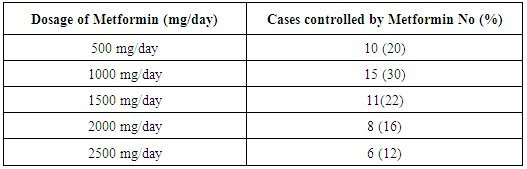
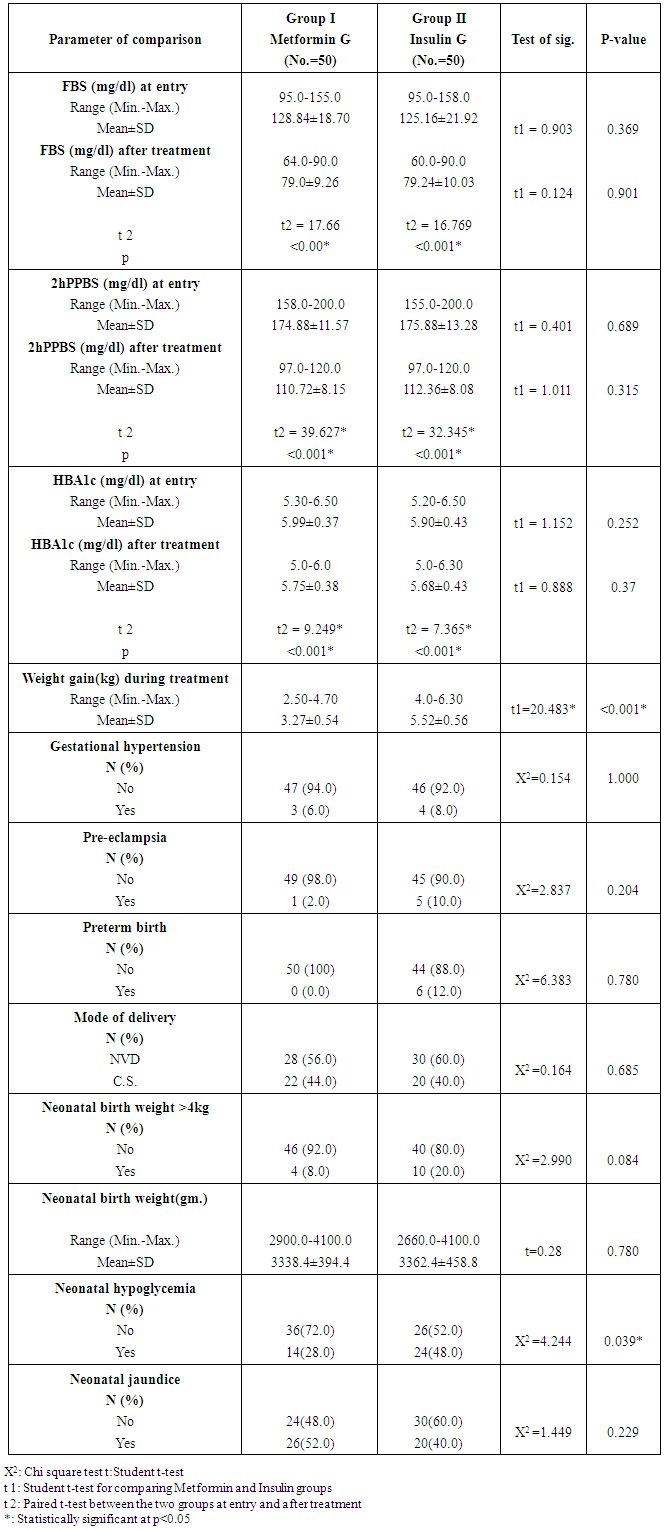
- There were no significant differences between the two studied groups as regard age (P= 0.183), gravidity (P=0.608), parity (P=0.699) and abortion (P=0.142), Gestational age at entry (P= 0.491), BMI at early pregnancy (P=0.346), and BMI>30 during treatment (P=0.216).Dosage of Metformin (β-Bigunide) at oral dose 500 mg daily in the morning and will be increased when necessary by 500mg weekly up to total dose 2500mg/day. Patients who achieve control by Metformin will be given insulin 24hs before planned delivery or will be given insulin when urgent delivery is needed to avoid neonatal hypoglycaemia.Regarding pattern of blood sugar in the studied groups, both metformin and insulin produced the same effect (p=0.901) on significant reduction of FBS after treatment (P<0.00 and 0.001) respectively. The same was found concerning 2hPPBS and HBA1c as the drugs used [metformin and insulin] had no difference in their effects on significant reduction of these parameters after treatment (P=0.252 and 0.37) respectively. Concerning maternal complications, gestational hypertension was found in (6.0%) and (8.0%) only of patients with Metformin and insulin groups correspondingly, there were no significant differences between them (P= 1.000). In addition preeclampsia were found in (2.0%) and (10.0%) for Metformin and insulin groups respectively, with no significant difference between the two studied groups (P=0.20). Preterm delivery less than 37 weeks were found in (12.0%) of insulin group and not found in Metformin group with no significant difference between the two studied groups (P=0.780). With reference to mode of delivery among patients of the studied groups C.S. were found in (44.0%) and (40.0%) of Metformin and insulin groups respectively. NVD was found in (56%) and (60%) of cases of Metformin and insulin groups respectively. There were no statistical significant differences between the two studied groups regarding mode of delivery (P=0.685). There were no significant differences between the two studied groups regarding birth weight (P=0.780) as neonatal birth weight ranged between 2900.0-4100.0 (gm.) and 2660.0-4100.0 (gm.) with the mean of 3338±394.4 (gm.) and 3362±458.8 (gm.) for Metformin and insulin groups respectively. Neonatal hypoglycemia occurred significantly (p=0.039) more among patients who treated with insulin (48.0%) whereas it was less in Metformin group (28.0%). Neonatal jaundice also occurred more among insulin (60.0%) than metformin (48.0%) cases, but with no significant differences between the two studied groups (P=0.229).
|
|
|
4. Discussion
- Gestational diabetes mellitus (GDM) is defined as ‘‘any degree of glucose intolerance with onset or first recognition during pregnancy”. [23] The incidence of GDM depends on the diagnostic criterion and varies widely in different racial groups. The incidence of GDM varies from 2 to 8%, according to ethnic background and the criteria used for diagnosis. [27] GDM associated with increased risk of a variety of maternal and perinatal complications including preeclampsia, cesarean section, macrosomia, shoulder dystocia, birth injuries, hypoglycemia, and respiratory distress syndrome. [28, 29] Several studies, including the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) and Metformin in Gestational Diabetes (MiG) trial have demonstrated that the risk of adverse pregnancy outcomes increases continuously with glucose levels, while it can be reduced with effective treatment of hyperglycemia. [6, 30] Attempts to prevent adverse pregnancy outcomes are based on recommendations for diagnosis and treatment by the American College of Obstetricians and Gynecologists (ACOG), and the American Diabetes Association (ADA). [31, 32]Traditionally, insulin therapy had been considered standard practice for women with GDM who could not have been controlled by medical nutrition therapy and physical activity. [14] Insulin therapy can be difficult for pregnant women due to multiple injection requirements, risk of hypoglycemia, and weight gain. Metformin is an alternative to insulin and is effective in the treatment of women with GDM. [33] It improves peripheral insulin resistance and decreases hepatic gluconeogenesis, and is not associated with weight gain or hypoglycemia. [34]Metformin readily crosses the placenta, exposing the fetus to concentrations approaching those in the maternal circulation. [35] A meta-analysis of pregnancy outcomes after first trimester exposure to Metformin did not show an increased risk of major malformations and other systematic reviews did not find substantial maternal or neonatal outcome differences with use of oral anti-diabetic agents compared with insulin in women with GDM. [36, 37] Follow up at age 18 months of 126 infants born to 109 mothers with PCOS who conceived on and continued Metformin during pregnancy reported similar size and motor-social development in the Metformin-exposed infants compared with infants of women not known to have PCOS. [38] Preliminary data from the follow up of 2-year-old children in the MiG trial showed no difference in body composition, diet and activity levels in the Metformin and insulin groups. [6] Although the long-term squeal of such exposure remains unknown, Metformin could potentially have effects on neonatal insulin resistance and obesity similar to those seen in adults. Although it crosses the placenta, Metformin appears to be safe in the second and third trimester of pregnancy. [18] The use of Metformin in pregnant women is still controversial; therefore, evaluation of the effect of Metformin and insulin in glycemic control and compared to pregnancy outcomes in GDM women were done. In the present study, one hundred women diagnosed with GDM were included. 50 women received insulin and the other 50 were treated with Metformin after failure of diet control and lifestyle advice for 2 weeks.There were no significant differences in baseline maternal characteristics including age, body mass index (BMI), parity, median gestational age at entry, HbAlc, mean fasting and 2-h post-prandial blood glucose levels. The mean fasting glucose value was 128.84 ± 18.70 mg/dl and mean 2-h postprandial value was 174.88 ± 11.57 mg/dl in the Metformin group compared to 125.16 ± 21.92 mg/dl and 175.88 ± 13.28 mg/dl respectively, in the insulin group on 75 g OGTT. In this randomized study, the mean blood glucose level at overnight fasting and postprandial and HbAlc level at delivery were similar in both groups throughout GDM treatment. The number of patients who reached the goal of an approximately equal fasting and postprandial glycaemia did not differ significantly between the two groups. Consequently, Metformin was found to be effective in the management of gestational diabetes mellitus.The Metformin in Gestational Diabetes (MiG) trial [6], a large randomized controlled trial conducted in Australia and New Zealand, compared Metformin (maximum daily dose of 2500 mg) with insulin in 751 women with GDM whose fasting glucose exceeded 97 mg/dL or had at least two 2-hour postprandial values exceed 121 mg/dL. Fasting and 2-hour postprandial capillary glucose levels were similar in the two groups during the 2 weeks prior to delivery; however, during the entire treatment period postprandial glucose levels averaged 112 mg/dL in the Metformin group and 115 mg/dL in the insulin group, a statistically but not clinically significant difference. Niromanesh S et al. [39] found that the two groups were similar in mean FBS (P=0.68) and postprandial measurements (P=0.87) throughout GDM treatment.Rai et al. [40] found that, though the values in the insulin group before therapy appear marginally higher, the difference was not statistically significant (P= 0.08-0.69; 95% Cl, 1.04-26.2). A significant reduction in adjusted mean glucose levels in the Metformin group was noted as compared to the insulin group (P = <0.001-0.003; 95% Cl, 5.2-29.7) after 1 week of therapy. Twelve (40%) patients on Metformin and 18 (69%) patients on insulin (Pearson X2=4.29; P= 0.038) required repeated dose adjustments based on glycemic profile. Metformin thus showed more uniform control of sugars as compared to insulin. Like the majority of studies, their study was prospective and not a randomized controlled trial, and the number of participants was small. In controversy with our results, Coetzee et al. tried Metformin in 60 diabetic patients, majority of them being maturity onset and only 21 were GDM. The failure rate in the former was 54% while in the latter it was 29%. This may be because 79% of their patients were overweight. [41]In another study, done by Ija's H et al. [17] 32% of the women on Metformin required additional insulin to reach normoglycaemia. Women with a need for supplemental insulin in that study were more obese, had higher fasting blood glucose concentrations in OGTT and needed medical treatment earlier than women who reached normoglycaemia with Metformin, suggesting that they exhibited a more severe insulin resistance. They also needed higher insulin doses to reach normoglycaemia than the women in the insulin group.Gestational hypertension was found in 3 (6.0%) and 4 (8.0%) for Metformin and insulin groups respectively, there were no statistical significant differences between the two studied groups (P= 1.000). Preeclampsia were found in 1 (2.0%) and 5 (10.0%) for Metformin and insulin groups respectively, insulin group have preeclampsia more than Metformin group but there were no statistical significant differences between the two studied groups. (P= 0.204).These findings are inconsistent with the study done by Hellmuth et al. [42] in a combined cohort of GDM and type 2 diabetes mellitus, as they observed increased rates of preeclampsia and perinatal loss in mothers treated with Metformin. [40] There was no observation of increased incidence of preeclampsia and no perinatal death with Metformin in our study. Their study was also retrospective, and control groups were inadequately matched, and the Metformin group had other increased risk factors for preeclampsia such as older age and obesity. The other studies did not find these complications to be an issue. [43, 44]Balani J, [21] found that preeclampsia occurred more frequently in the insulin group, but the difference did not achieve statistical significance (P = 0.06). However, the incidence of preeclampsia was statistically less frequent if the 13 patients who didn't achieve glycemic control on Metformin and required additional insulin are included in the Metformin group (P = 0.02). Rates of pregnancy-induced hypertension were similar in the two groups. It is now believed that Metformin may reduce preeclampsia in GDM women by reducing the endothelial activation and maternal inflammatory response of insulin resistance. [43]Niromanesh S et al [39] found that PIH was considerably less frequent in the Metformin group than the insulin group, although it did not reach statistical significance (5%) versus (13.8%), respectively, (P = 0.058).In the present study preterm delivery less than 37 weeks were found in 6 cases (12.0%) for insulin group only. This was found to be statistically insignificant. This is in contrast to the MiG trial, where preterm births were significantly higher in the Metformin group (12.1%) vs. (7.6%), (P = 0.04). [6] There was no obvious explanation for that finding. In the MiG trial, the rate of iatrogenic preterm births was not different between the Metformin and insulin groups, but there was a trend to more spontaneous preterm births in the Metformin group. Other studies reporting outcomes in women treated with Metformin have not seen an increase in preterm deliveries. [6, 17, 21, 39] Overall, these data suggest that Metformin does not have a specific effect on the risk of preterm birth, but further randomized data would be of interest.Niromanesh S et al [39] found that the incidence of preterm delivery was twofold higher in the Metformin group but this was not significantly different. They said that this inconsistency could be due to chance or unrecognized effect of Metformin on the labor. On the contrary, Balani [21] previously showed that preterm delivery was more common in the insulin group. Gandhi P et al. [45] like Balani J et al. [21], found that mean gestation age at birth was statistically higher in the Metformin group (38.7±1.31) weeks than insulin group (38.3±1.9) weeks. Comparison between the two studied groups regarding weight gain during pregnancy showed that, the mean of 3.27 ± 0.54 (kg) and 5.52 ± 0.56 (kg) for Metformin and insulin groups respectively, insulin group have weight gain values statistically higher than Metformin group (P <0.001). Similar to this study findings, Niromanesh S et al. [39] found that the insulin group had a statistically significantly higher total and after entry weight gain [13.7 (+3.1) and 4.5 (+1.7) kg] than the Metformin group [11.3 (+3.8) and 3.3(+1.4) kg] (P< 0.001). Also Rowan et al. (43) demonstrated that Metformin treatment was associated with lower maternal weight gain, which is especially beneficial in women with GDM, who are likely to be overweight or obese and are at risk of gaining more weight as pregnancy advances. Also J. Balani et al [21] found that Metformin-treated women gained less overall weight from the time of enrolment until the time of delivery whatever the gestational age of starting treatment (0.05 vs. 2.15 kg; P < 0.01). When treatment had been initiated at 30-31 weeks’ gestation, weight gain was significantly reduced in the Metformin group compared with insulin.In the current study C.S. were found in 22 (44.0%) and 20 (40.0%) for Metformin and insulin groups respectively, there were no statistical significant differences between the two studied groups, (P=0.685). There is no difference in the mode of delivery between the 2 groups; this is in agreement with most authors. Some have reported a cesarean delivery rate less than (25%) with Metformin treatment. [46, 47] The rate (70%) of cesarean deliveries in some studies done by Silva JC, et al [45] should not be entirely attributed to GDM. Such high rates are common in some low resource countries, including Brazil, which has led governments to implement programs promoting vaginal delivery. [48] Ija's H et al. [17] found that there were more caesarean deliveries (38%) in the Metformin group than in the insulin group. The increase of the caesarean section rate in the Metformin group was a result of an increased incidence of intra labor caesarean sections for prolonged labor or presumed fetal compromise. In another study by Gandhi P et al. [43] there was no significant difference in maternal outcomes between the Metformin and non-Metformin groups in caesarean section rate, preeclampsia and gestational hypertension.In the present study the mean birth weight of the newborns did not differ significantly between the Metformin and insulin groups, as the mean birth weight was 3338.4±394.4 (gm) and 3362.4±458.8 (gm) for Metformin and insulin groups respectively, there were no statistical significant differences between the two studied groups (P=0.780). There were 10 cases (20%) with birth weight more than or equal to 4kg, (macrosomia) in the insulin group and 4 cases (8%) with that weight in the Metformin group, (P=0.084).In contrast to other study done by Gandhi P et al, [45] women in the Metformin group had a significantly lower incidence of macrosomia (birth weight>4kg) (8.2% vs. 14.3%), as well as birth weight >90th centile (14.8% vs. 23.7%).In the MiG trial [6] there was no significant difference in the proportion of large for gestational age (birth weight >90th centile) neonates in the Metformin group vs. insulin group. It is likely that the high supplementary insulin use (46.3%) in Metformin group to achieve stringent glycaemic target was responsible for this in the MIG trial. In another observational study by Balani et al. [21], which compared pregnancy outcomes between Metformin and insulin groups, both macrosomia and large for gestational age babies were less in Metformin group, but the result was not significant.In Niromanesh S et al study, [39] the relative risk of LGA (birth weight > 90th percentile) in the Metformin group was half that of the insulin group (RR: 0.5, (95%) Cl: 0.3-0.9, P= 0.012). There was only one case in the insulin group with a birth weight more than 4500 g. Three neonates (3.8%) from the Metformin group versus 8 (10.0%) from the insulin group were macrocosmic (birth weight ≥ 4000 g) (P= 0.118). Similarly Gandhi P et al. (43) found that women in the Metformin group had a significantly lower rate of macrosomia (birth weight >4 kg) (8.2% vs. 14.3%), as well birth weight >90th centile (14.8%) vs. (23.7%).Hypoglycemia were found in 14 (28.0%) and 24 (48.0%) of Metformin and insulin groups respectively with statistical significant differences (P=0.039). The results from the current study are consistent with those obtained by Tertti et al. [44] their study found significantly fewer neonates with hypoglycemia in the Metformin group. The study was a retrospective case-control that was not randomized, and control groups were inadequately matched; therefore, it is probable that insulin-treated patients were slightly more hyperglycemic than those in the Metformin group. In another study done by Ija"s H et al. [17] the frequency of neonatal hypoglycemia, neonatal Hyperbilirubinemia were slightly but not significantly higher in the insulin group.The primary composite outcome in the MiG trial (neonatal hypoglycemia, respiratory distress, need for phototherapy, birth trauma, 5- minute APGAR score < 7, prematurity) did not differ between the two treatment groups (32% in each). Secondary outcomes, such as LGA, cord insulin levels, and subcutaneous fat thickness, were similar. A secondary outcome, severe neonatal hypoglycemia (any blood glucose less than 28.8 mg/dL) was more common in the insulin group (8%) than in the group of women who were randomized to Metformin (3%). [43]Jaundice was found in 26 cases (52.0%) and 20 cases (40.0%) of Metformin and insulin group respectively, there were no statistical significant differences between the two studied groups (P=0.229). Coetzee et. al. [41] noted a higher incidence of neonatal Hyperbilirubinemia in the Metformin group though overall neonatal morbidity was low.The present study showed that Metformin is a safe and clinically relevant medical alternative for treating GDM. The incidence of adverse pregnancy or neonatal outcomes was not increased in women treated with Metformin compared with women treated with insulin. However, inconsistencies in clinical outcomes measured across studies and lack of enough data on the comparative risks and benefits of Metformin for GDM have made it difficult to draw firm conclusions. Further studies with larger volume involving high-risk populations are recommended.
5. Conclusions
- Metformin is effective as insulin for the management of GDM. Metformin can be used securely during pregnancy as it is not linked with congenital malformations or increased maternal or neonatal complications. Metformin decreases the rate of neonatal hypoglycemia. Insulin is still the gold standard for management of gestational diabetes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML