-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Obstetrics and Gynecology
p-ISSN: 2326-120X e-ISSN: 2326-1218
2015; 3(1): 8-12
doi:10.5923/j.rog.20150301.03
Impact of High-fat Environment on Ovarian Androgen Synthesis in Rats and the Associated Pathophysiological Changes
Cong L. , Ying C. , Lian-Lian W. , Xiaoqiu X.
O&G department, the First Affiliated hospital of Chongqing medical university, Chongqing, China
Correspondence to: Cong L. , O&G department, the First Affiliated hospital of Chongqing medical university, Chongqing, China.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Objective: To analyze the pathophysiological changes of ovary under high-fat environment, determine the effects of hyperlipidemia on polycystic ovary, and explore the intrinsic relationship between the hyperlipidemia and hyperandrogenism. Method: Young female rats were randomly divided into high-fat diet and control groups. Till sexual maturity, the high-fat diet group was fed with 45% high-fat diet than the control group. The dynamic changes in biochemical markers such as blood glucose and sex hormone of high-fat diet group were measured. The ovarian tissues were isolated and hematoxylin and eosin staining and electron microscopy were used to detect the ovarian ultrastructure variations. Variations in the levels of estrogen receptor, progesterone receptor, and androgen receptor were measured to analyze the possible influence of high-fat diet on ovarian androgen synthesis. Results: In the high-fat diet group, only immature ovarian follicles in large quantity were found and the number of atretic follicles significantly increased. Immunohistochemical studies showed that in the high-fat diet group, expression of ovarian androgen receptor in the theca cell significantly enhanced and expression of the estrogen receptor was slightly elevated. However the level of three receptors mildly elevated in the granulosa cells, but when compared with the control group this difference was not significant. Conclusions: High-fat feeding may promote mature follicles disorder in the ovarian tissues, the theca cell morphology showed the prevalence of hyperfunction, and the expression of androgen receptor significantly increased, providing a reliable pathophysiological basis for the androgen synthesis.
Keywords: High-fat feeding, Ovary, Androgen receptor, Theca cells
Cite this paper: Cong L. , Ying C. , Lian-Lian W. , Xiaoqiu X. , Impact of High-fat Environment on Ovarian Androgen Synthesis in Rats and the Associated Pathophysiological Changes, Research in Obstetrics and Gynecology, Vol. 3 No. 1, 2015, pp. 8-12. doi: 10.5923/j.rog.20150301.03.
1. Introduction
- Polycystic ovary syndrome (PCOS), the most common endocrine systemic disease resulting in abnormal glucose and lipid metabolism in women of childbearing age, is one of the main causes of infertility [1]. Occurrence and development of PCOS is closely related to three main components: hyperandrogenism, insulin resistance, and lipid metabolism disorder, of which hyperandrogenism is of utmost importance. However, when the correlation between the other two components and hyperandrogenism is revealed, the cure of PCOS becomes possible. Though, recent studies have fully described the correlation of insulin resistance and hyperandrogenism, no serious attention has been paid on or in-depth researches have been conducted regarding the inherent correlation between lipid metabolism disorder and hyperandrogenism [2]. Epidemiological similarities between obesity and PCOS trigger the reciprocal appearance of hyperlipidemia and hyperandrogenism, which may play an important part in occurrence and development of PCOS, but the interaction mechanism, is still unknown. Identifying the interaction mechanism helps to improve the efficacy of clinical management of PCOS.Since lipid is a raw material for synthesis of steroid hormones, excessive intake of high-fat diet will certainly affect the synthesis and secretion of the androgen in the theca cells. Some studies have found that lipid peroxidation stress medications such as vitamin E can induce apoptosis of thecal cells in rats, and reduce androgen synthesis [3]. Lipid lowering agents like statins can significantly inhibit hyperthecosis of the theca cells and change the androgen secretion [4]. Androgen receptor, mainly located in the theca cells under the physiological conditions, is an important site for the androgen as it plays an important role in the human body. Hyperfunction of the androgen receptor indicates increased androgen synthesis, while its scattered expression in the granulosa cells is mainly associated with the transformation process of androgen to estrogen. Elevation of androgen may result in abnormal pathophysiological changes such as ovulation disorder. Synthesis and functionality of the progesterone mainly occur in the granulosa cells. Strong granulosa cell function may be expressed as enhanced activity of the progesterone receptors. Ovarian tissue is a possible target for lipid peroxidation loss, its abnormal changes under the high-fat environment will certainly affect the synthesis and secretion of hormones in the body, resulting in abnormal pathophysiological changes. To illustrate the theoretical basis of this possibility, we described a regulatory aspect of the androgen synthesis in the theca cells, to establish an intrinsic relationship between the metabolism and hyperandrogenism and thus provide an etiological basis for PCOS.
2. Materials and Methods
- SD rats model with hyperlipidemia A total of 40 female SFP-level SD 4-week-old weaned rats were selected and randomly divided into two groups, namely general feeding and high-fat diet group (60% calories from fat). They were fed for 12 weeks under the same conditions, all were in good condition, and no rat died. Their weight was recorded weekly, and overall results showed that the weight gain of the high-fat diet group was higher than that of the control group. The high-fat diet was a standardized product of The Shanghai Slac Laboratory Animal Pvt. Ltd. The animal experiments were approved by the Animal Care and Use Committee of Chongqing Medical University. Analysis of serum biochemical parameter Rats were anaesthetized using chloral hydrate, and 0.5 ml of blood was collected from the ear vein. Enzyme linked immunosorbent assay (ELISA) was used to measure blood lipid content of each group every week, starting from sexual maturity (10-week-old).
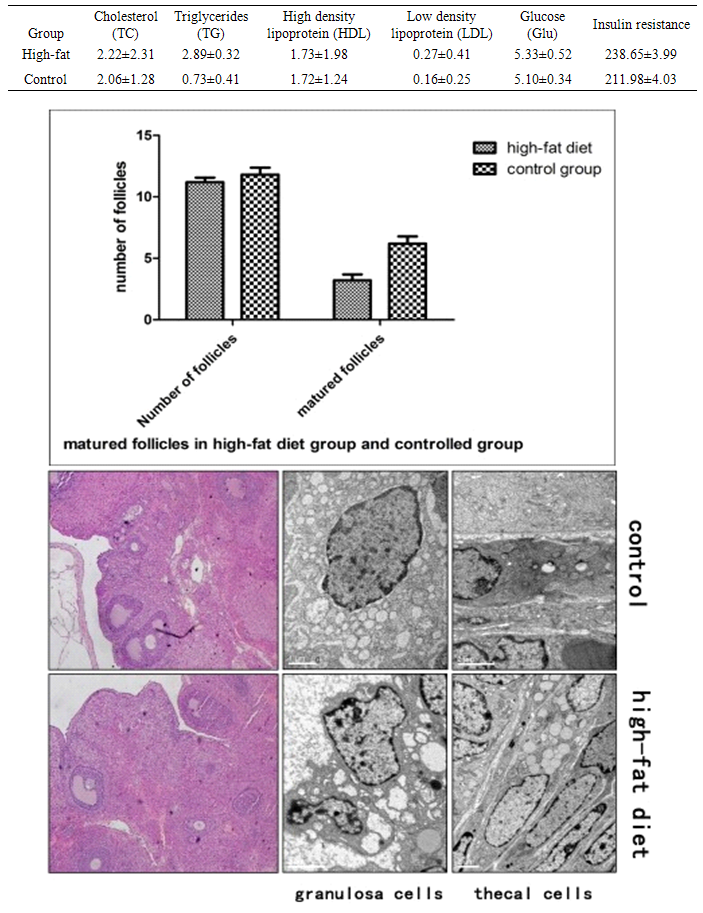 | Figure 1. Changes in the follicular levels and morphology of granulosa cells and theca cells |
3. Results
- Changes of blood lipid and blood glucose levelFrom Table, it can be observed that in the high-fat diet group there is an increase in the concentration of serum triglycerides compared with the control group. The variations in the level of cholesterol, high density lipoprotein, low density lipoprotein, fasting insulin resistance, and glucose of the high-fat diet group was not significant when compared to the control group (P > 0.05). This result excluded the interference of hyperinsulinemia on the experimental results and was in-line with correlation analysis between the hyperlipidemia injury factor and triglycerides independent factors. These results indicated the success of employing animal models in the present study. Ovarian tissue morphology under high-fat environmentHE staining showed that the number of matured follicles of the high-fat diet group was significantly less than that of the control group (P < 0.05). A large number of atretic follicles were observed in high-fat diet group indicating that the ovulation function was affected. There was a reduction of 30% in the number of the matured follicles in the high-fat diet group indicating significant difference between the two groups (P < 0.05). Electron microscopy showed a clear difference between the thecal cells and the granulosa cells, where granulosa cells were arranged in a cubic shape and the thecal cells in a spindle shape. In the high-fat diet group, the organelle and lipid bodies in the thecal cells significantly increased and the organelle was in good conditions without any pathological change. However, the granulosa cells showed significant pathological change under the high-fat environment, where the intracellular mitochondria underwent swelling and vacuolation, suggesting that the granulosa cell function was significantly impaired. Meanwhile, the theca cell function was more vigorous compared with the control group, which provided a structural basis for androgen synthesis of the theca cells. HE staining revealed that the ovary of the high-fat diet group contained one to two matured follicles, one corpus luteum after ovulation and a number of small atretic follicles. However, in the control group, matured follicles of different stages were present without the presence of obvious small follicles and atretic follicles. Scanning electron microscopy results showed that the granulosa cells of the control group were arranged in a cubic shape, and mitochondrial membrane was swollen, vacuolated, and decomposed, presenting obvious pathological changes. A small number of lipid droplet, along with scattered mitochondria was found inside the follicular cytoplasm. These two types of cells did not exhibit significant pathological changes. However, in the high-fat diet group, a large number of lipid droplets inside the follicular cytoplasm and increased number of mitochondria were observed. But the functional status was maintained and no mitochondrial membrane swelling, necrosis, and other pathological changes were observed. Immunohistochemical results of hormone receptor expression in ovarian tissueImmunohistochemical studies showed that in the control group, most of the estrogen receptor (ER) and progesterone receptor (PR) were expressed in the granulosa cells, while only a few expressions were observed in the theca cells. However, the androgen receptor (AR) expressions mainly took place in the theca cell and to a small extent in the granulosa cells. On the other hand, in the high-fat diet group, the ER and PR intensity in the granulosa cells and the theca cells were elevated in varying degrees with no statistical difference. However, AR expression in the theca cells was significantly enhanced. The semi-quantitative analysis of strong positive expression showed a significant difference compared with the control group (P < 0.05). The AR expression in the granulosa cells was mildly elevated with no significant difference.
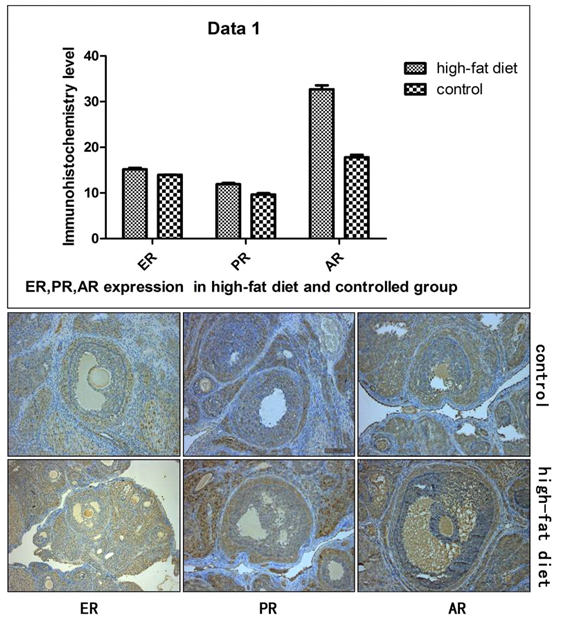 | Figure 2. Immunohistochemical results of ER, PR, and AR expression of the high-fat diet and control |
4. Discussion
- A large cellular intake of exogenous fatty acid, triglycerides, and cholesterol occurs in case of obesity and short-term overnutrition, which results in increased synthesis of fat cells. These excessively accumulated lipid droplets in cells may lead to tissue specific toxicity and eventually result in abnormal cell regulation and organ function impairment, namely cellular lipid toxicity [5]. Cellular accumulation of lipid droplets leads to increased free fatty acids and oxidative stress, and subsequently increased lipid peroxidation which is cytotoxic and highly reactive, this condition results in functional impairments of the organelle such as mitochondria and endoplasmic reticulum and causes functional disorder of cells [6]. The morphological results of the present study showed that under the high-fat environment, the ovarian granulosa cells suffered significant oxidative damage, which were presented as organelle damage. While in the theca cells, the number of mitochondria and other organelles increased thus presenting cell hyperactivity. Such difference in ovarian tissues suggests that there is a significant difference of cellular antioxidant function in high-fat environment and correspondingly presents abnormal hormone synthesis. The above morphological results provide a structural basis for the present molecular studies. So under the high-fat environment, the granulosa cells are significantly impaired, maturation of ovarian follicles is inhibited to a certain degree, while the theca cells which are the main cells for androgen synthesis maintain shape integrity and thus provide prospects for the follow-up study of their hyperfunction. In the theca cells, both the PR and AR exhibited enhanced activity, especially the activity of the latter was strongly positive. AR expression in ovarian tissue mainly occured in the nuclear membrane of the theca cells and a small amount of expression was observed in the granulosa cells. This experiment showed that in the high-fat diet group, a large amount of brown strong positive expression was observed around the nuclear membrane of the theca cells and a small amount of expression was observed in the granulosa cells, which was confirmed microscopically. In the control group, evenly distributed AR expression was observed, but the intensity was significantly weaker than that in the high-fat diet group, indicating that AR expression was significantly enhanced after high-fat feeding, which provides a structural basis for further studies of androgen hyperfunction. Mild increase of the progestogen may be due to the increased activity of precursor of androgen and the subsequently increased adaptability. The expressions of the three receptors in the granulosa cells did not present significant difference. Due to damage of the cell structures, the granulosa cells suffered peroxide stress, which led to ovarian follicle disorder and reduction in the number of dominant follicles which resulted in Doran-like changes of the ovary that was consistent with our morphological results. This clarification for expression difference of these cells helps to understand the androgen regulation under the high-fat environment. Our serological results confirmed that the high-fat diet group had increased concentration of triglycerides while it did not raise the level of glucose and insulin resistance and cholesterol which is the main raw material for synthesis of steroid hormones. These results indicate that under the high-fat environment, change of ovarian function is not due to hyperandrogenism, but due to other factors such as peroxidation injury [7]. Though, hyperlipidemia is not the root cause for hyperandrogenism and polycystic ovary, it does have a significant impact on ovulatory function and endocrine function. Under the high-fat environment, androgen synthesis in the theca cells is significantly enhanced, while ovary undergoes polycystic changes. The abnormal pathophysiological changes in the function of ovary clarify the intrinsic relationship between hyperlipidemia and hyperandrogenism.
5. Conclusions
- High-fat feeding may promote mature follicles disorder in the ovarian tissues, the theca cell morphology showed the prevalence of hyperfunction, and the expression of androgen receptor significantly increased, providing a reliable pathophysiological basis for the androgen synthesis.
Funding
- This project was funded by the National Natural Science Foundation Youth Fund (No. 81100399) and Chongqing Science Committee (No. cstc2011jjA0111).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML