-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Obstetrics and Gynecology
2012; 1(3): 30-35
doi: 10.5923/j.rog.20120103.04
Asymptomatic Complete Placenta Previa: A Case Report and Review of Literature
Genovese F. 1, Marilli I. 1, Benintende G. 2, Famà A. 1, Vizzini S. 1, Carbonaro A. 1, Palumbo M. 1, Pafumi C. 1
1Institute of Obstetric and Gynecologic Pathology, Department of surgery, University of Catania, Italy, chair: Prof. Giuseppe Zarbo
2Complex Operative Unit of Prenatal Diagnosis and Medical Genetics, University Hospital , Vittorio Emanuele, Catania, Italy
Correspondence to: Pafumi C. , Institute of Obstetric and Gynecologic Pathology, Department of surgery, University of Catania, Italy, chair: Prof. Giuseppe Zarbo.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
We present a case of a stable and asymptomatic complete placenta previa for all the duration of pregnancy in a 40-year-old woman, treated with an elective cesarean section at 37 weeks of gestational age. Placenta previa is a condition derived from an abnormal implantation of the embryos in the lower uterine segment. Risk factors for the development of placenta previa include prior cesarean delivery, pregnancy termination, intrauterine surgery, smoking, multi-fetal gestation, increasing parity, maternal age and the rising rates of cesarean section. Usually complete placenta previa becomes symptomatic in third trimester of gestational age and it is associated with adverse consequences for both mother and children, such as intra-uterine growth restriction, preterm birth, antenatal and intra-partum hemorrhage, maternal blood transfusion and emergency hysterectomy. In this article we performed a review the international literature of the last twenty years of similar cases, emphasizing on the aspects of the management and time of delivery in patient with placenta previa, and in particular we focus on the management of stable and asymptomatic cases. On the basis of our experience, the risks for both mother and fetus and the results of the literature, we conclude that in presence of a stable and asymptomatic complete placenta previa an early term birth (ETB) at 37 weeks of gestational age, rather than a late preterm birth (LPTB) between 34-37 weeks, is a more appropriate time of delivery, and it is associated to a better prognosis for both mother and child.
Keywords: Placenta Previa, Hemorrhage, Caesarean Section, Lower Uterine Segment
Article Outline
1. Background
- Antepartum hemorrhage complicates 2-5% of pregnancies, which approximately one-third is due to placenta previa, a condition derived to an abnormal implantation of the embryos in the lower uterine segment[1]. Placenta previa has been classified by the degree of encroachment upon the internal cervical os[2]. In placenta previa hemorrhage is more likely to occur during third trimester, as a consequence of the development of the lower uterine segment and of the dilation of the cervix due to uterine contractions; also, vaginal examination may lead to an antepartum hemorrhage. Risk factors for the development of placenta previa include prior cesarean delivery, pregnancy termination, intrauterine surgery, smoking, multi-fetal gestation, increasing parity, maternal age and the rising rates of Cesarean section[3]. Placenta previa is associated with adverse consequences for both mother and children, such as intra-uterine growth restriction (IUGR), preterm birth, antenatal and intra-partum hemorrhage, maternal blood transfusion and emergency hysterectomy[4].Classically placenta previa is divided in different forms:▪ Marginal: the placenta is next to cervix but does not cover the intern cervical os.▪ Partial: the placenta covers partly the intern cervical os.▪ Complete: the placenta covers completely the intern cervical osIt is estimated placenta previa has been diagnosed increasingly in recent decades, due mostly to the widespread use of ultrasound to occur in between 0.2 and 0.3% of third-trimester pregnancies. False-positive diagnoses are common in the second trimester and the term “potential placenta previa” is used to describe this situation[5]. In some cases of mid-trimester potential placenta previa is more likely to migrate (phenomenon of the “rising” placenta) and ultrasound may be useful to predict this process[6]. Apart from ultrasound a valid imaging modality to study and investigate placenta in antepartum period seems to be magnetic resonance (MR). MR imaging compared with US may be superior in some settings, especially it improves soft-tissue contrast and wider field of view; however, it is limited by cost, patient claustrophobia, and limited availability of both imaging unit technology and skilled image interpretation[7]. About its safety, MR, as well as US, does not use ionizing radiation and appears to be a safer modality than computed tomography (CT), even if fetal risks remain uncertain compared to ultrasound. MR imaging allows identification of the position of placenta. However, it has been demonstrated to be less specific than color Doppler flow imaging in the diagnosis of placenta previa[8], but it can add diagnostic value when further characterization is required, particularly in the setting of invasive placental processes such as placenta accreta and gestational trophoblastic disease[9].
2. Case report
- A 40- year-old woman with three pregnancies, two previous miscarriages at 7 and 8 weeks of gestational age in the past two years, was admitted in our institute at 37 weeks. She weighed 71 Kg and was tall 170 cm. She was heterozygous for the mutation G1691A for the gene of Leiden’s factor and homozygous for the mutation C677T of MTHFR’s gene. She was treated with levotiroxin 50 mg for the presence of multiple thyroid nodules. In the second trimester the routine ultrasound suspected a complete placenta previa, subsequently confirmed at the transvaginal ultrasound performed at the third trimester by the presence of a placental edge overlapping the internal os (complete placenta previa) (Picture 1). The cervical length was >30mm. For all the pregnancy long the patient was stable and asymptomatic. During pregnancy the patient was treated with acetyl salicylic acid (suspended two days before the delivery), prenatal vitamins, and with antenatal corticosteroids (betamethasone 4 mg).
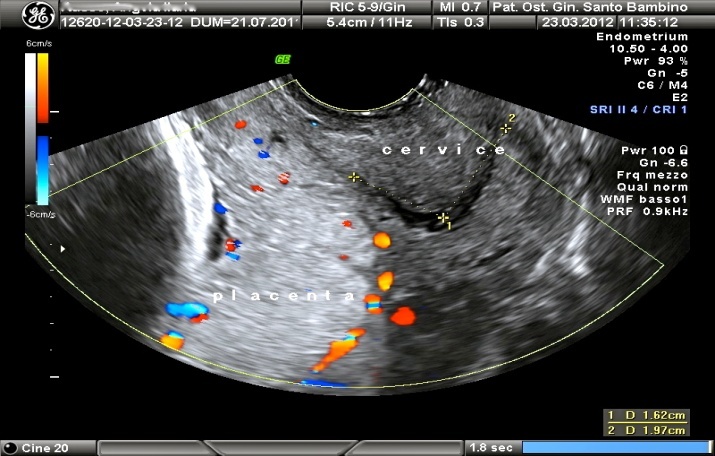 | Picture 1. ultrasonographic image of the complete placenta previa at 33 weeks of gestational age |
 | Picture 2. lower uterine segment previous the incision |
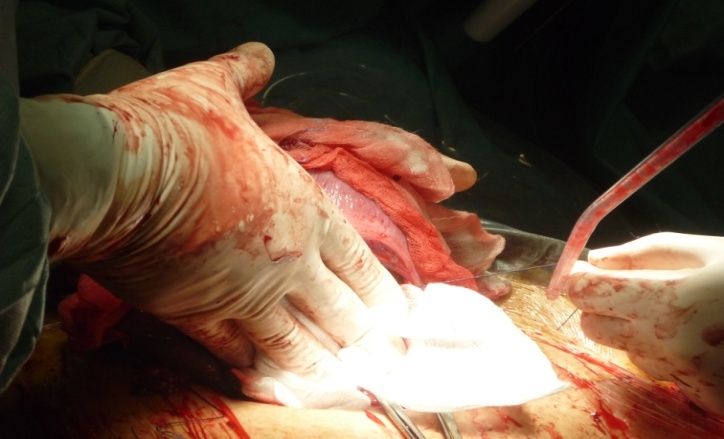 | Picture 3. external mechanical compression of the lower uterine segment |
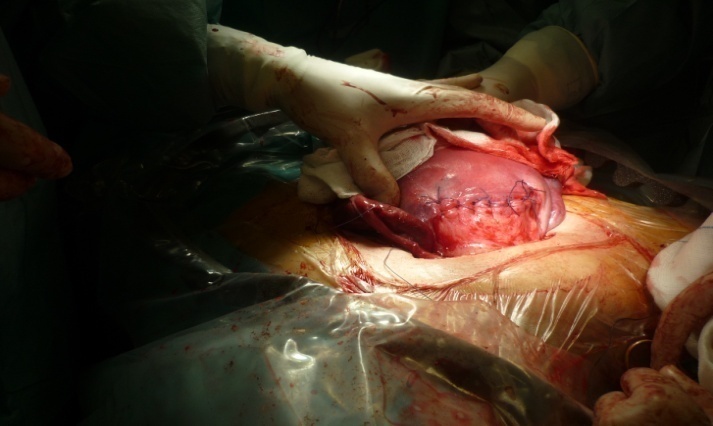 | Pictures 4. suture of the uterus in double layer |
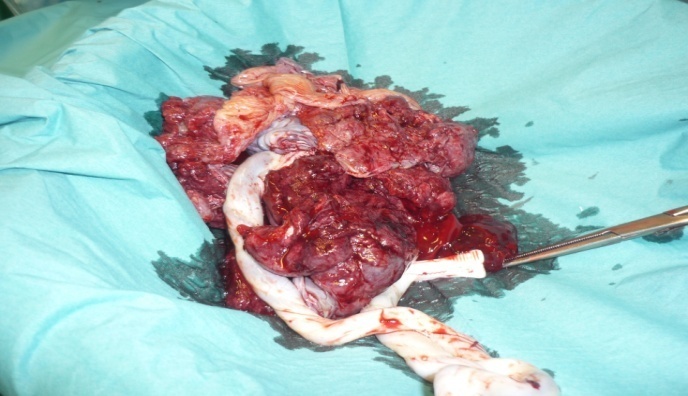 | Picture 5. the placenta after the manual removal. Necrotic spots are evident |
3. Diagnosis and Management
- Although around 5% of women have ultrasound evidence of a low placenta at 16 to 18 weeks, only 10% of this 5% (i.e. 0.5% overall) actually have a placenta previa at delivery. The apparent change of placental position results from formation of the lower uterine segment[10].A conclusive diagnosis of complete placenta previa is ascertained sonographically in the third trimester, when upward migration from the internal cervical os becomes unlikely[11, 12, 13]. Transvaginal ultrasound, if available and well established, is preferred to transabdominal sonography for the diagnosis of placenta previa. There are a good number of potential theoretical advantages to the use of transvaginal ultrasound in this situation; imaging is better and the woman does not need a full bladder, thus avoiding both maternal discomfort and also distortion of the anatomy of the lower uterine segment and cervix. The major problem with this technique is represented by the insertion of the probe into the vagina of a woman with possible placenta previa that may provoke bleeding. Advocates of transvaginal ultrasound argue that the probe should be inserted no more than three centimeters into the vagina and should not therefore come into contact with the cervix or lower segment, and that the improved images outweigh the theoretical disadvantages of provoking bleeding[10].A placental edge exactly reaching the internal os is described as 0 mm. When the placental edge reaches or overlaps the internal os on transvaginal sonogram (TVS) between 18 and 24 weeks’ gestation (incidence 2-4%), a follow-up examination for placental location in the third trimester is recommended. Overlap of more than 15 mm is associated with an increased likelihood of placenta previa at term (ll-2A). When the placental edge lies between 20 mm away from the internal os and 20 mm of overlap after 26 weeks' gestation, ultrasound should be repeated at regular intervals depending on the gestational age, distance from the internal os, and clinical features such as bleeding, because continued change in placental location is likely. Overlap of 20 mm or more at any time in the third trimester is highly predictive of the need for Caesarean section (CS) (llI-B). The os-placental edge distance on TVS after 35 weeks’ gestation is valuable in planning route of delivery. When the placental edge lies > 20 mm away from the internal cervical os, women can be offered a trial of labor with a high expectation of success. A distance of 20 to 0 mm away from the os is associated with a higher CS rate, although vaginal delivery is still possible depending on the clinical circumstances (ll-2A). In general, any degree of overlap (> 0 mm) after 35 weeks is an indication for Caesarean section as the route of delivery (ll-2A). Outpatient management of placenta previa may be appropriate for stable women with home support, close proximity to a hospital, and readily available transportation and telephone communication (ll-2C). There is insufficient evidence to recommend the practice of cervical cerclage to reduce bleeding in placenta previa (llI-D). Regional anesthesia may be employed for CS in the presence of placenta previa (II-2B). Women with a placenta previa and a prior CS are at high risk for placenta accreta. If there is imaging evidence of pathological adherence of the placenta, delivery should be planned in an appropriate setting with adequate resources (II-2B)[14].Bahar et al in 2009 conducted a retrospective study over a 10-year period from 1996 to the end of 2005 including 306 women presenting different types of placenta previa (PP). The authors compared risk factors and pregnancy outcome in different types of PP. The overall incidence of PP was 0.73%. Major PP (complete or partial) occurred in 173 women (56.5%) and minor PP (marginal PP pr low-lying placenta) in 133 women (43.5%). There were no differences between women with major and minor PP regarding age, parity, and previous miscarriages. After controlling for confounding factors, women with major PP showed a significantly higher incidence of antepartum hemorrhage (OR 3.18; 95% CI 1.58-6.4, P=0.001), placenta accreta (OR 3.2; 95% CI 1.22-8.33, P=0.017), and hysterectomy (OR 5.1; 95% CI 1.31-19.86, P=0.019). Antepartum hemorrhage in women with PP was associated with premature delivery (OR 14.9; 95% CI 4.9-45.1, P<0.001), more commonly in women with major PP. The only significant difference between women with major and minor PP regarding neonatal outcome was that major PP was associated with higher incidence of admission to the neonatal intensive care unit (P=0.014). They concluded that complete or partial placenta previa is associated with higher morbidity than minor PP.[15]. Even if placenta previa often requires iatrogenic preterm (PTB) <34 weeks because of maternal bleeding or spontaneous preterm labor, mostly because catastrophic bleeding can occur and is not predictable on the basis of clinical factors, there is also a good number of women that remain asymptomatic. In these cases clinicians must decide when to schedule cesarean delivery in a “stable” patient with placenta previa[16, 17, 18, 19].In their U.S. population-based study Ananth et al discovered that <34 weeks PTBs in women with placenta were only 16.9%, 27.5% women delivered between 34-37 weeks, and 55.6% occur ≥37 weeks[19]. Another important predicting factor in women with placenta previa is cervical length (CL). Fukushima et al conducted a recent study on eighty uncomplicated, singleton pregnancies with an antenatal diagnosed of placenta previa. The aim of this study was to evaluate the relationship between cervical length (CL) and obstetrical outcomes in women with placenta previa. N=60 women had a CL ≥30 mm, n=20 women had a CL <30 mm. The mean CL was 38.5±5.4mm and 26.9±3.2mm and the mean gestational age at measurement was 29.2±2.7 and 28.5±2.7weeks of gestation for the longer and shorter CL groups, respectively. The median estimated blood loss at cesarean section (CS) was significantly higher in the shorter CL group (1302mL vs. 2139mL, P=0.023) as was the percentage of patients with massive intraoperative hemorrhage (60.0 vs. 18.3%, P=0.001). In the shorter versus longer CL patients, emergent CS before 37weeks (23.3 vs. 50.0%, P=0.046) and the percentage of patients with placental adherence (6.7 vs. 35.0%, P=0.004) were both significantly more frequent in the shorter CL group. The shorter CL was a risk factor both for massive estimated blood loss (≥2000mL) (odds ratio 6.34, 95% confidence interval 1.91-21.02, P≤0.01) and placental adherence (odds ratio 6.26, 95% confidence interval 1.23-31.87, P≤0.05) in the multivariate analysis[20]. Stafford et al in 2010 reached to similar conclusion. They performed transvaginal cervical-length measurement on all singleton gestations with placenta previa (N=89) at or beyond 24 weeks of gestation. Of these, 68 women (76%) had placenta previa at delivery; 49 women (43%) with placenta previa had CL ≤30 mm. Women with previa and a short cervix were more likely to require delivery for hemorrhage, 79% compared with 28%, and to deliver preterm, 69% compared with 21% (both P<.001). Tocodynamometer evidence of regular uterine contractions was more common with a short cervix than with a longer cervix, 69% compared with 21% (P<.001). Conversely, 64% with a cervical length greater than 30 mm had no bleeding episodes and progressed to term. The authors concluded women with placenta previa and cervical length of 30 mm or less were three times more likely to deliver preterm than those with longer cervices. Moreover, women with previa and a short cervix were more likely to develop bleeding. Antepartum hospitalization for vaginal bleeding was more than twice as common among women with cervical length 30 mm or less as was the risk of delivery for hemorrhage. Although vaginal bleeding with placenta previa has traditionally been considered painless, in this study more than one third of women with a short cervix who presented vaginal bleeding experienced perceptible contractions as compared with only 3% of women who had a longer cervix[16]In asymptomatic women with placenta previa clinician should considered the potential advantage of a schedule late preterm birth (LPTB) between 34 and 37 weeks. The primary advantage of earlier delivery is that it decreases the probability of a woman presenting with acute hemorrhage and the recourse to emergent cesarean delivery. The acute hemorrhage with its catastrophic blood loss can rapidly lead to cardiovascular failure, hypovolemic shock, disseminated intravascular coagulopathy (DIC) and fetal compromise. Many authors noticed when the patient is stable and delivery is performed under optimal circumstances, in a tertiary care hospital with ready availability of resources (blood bank, Intensive Care Unit, Neonatal Intensive Care Unit) the risk of complications for both mother and child may be lower[17]. Robinson et al in 2010 conducted an analysis to determine the optimal gestational age at which to deliver individuals with ultrasonographic evidence of both placenta previa and accreta in individuals who reach 34 weeks of gestation[21]. They compared nine different strategies for the timing of delivery in these women. Strategies 1 and 2 included delivery at 34 and 35 weeks, respectively, 1 week after previous administration of antenatal corticosteroids for neonatal benefit. The remaining strategies did not involve routine administration of ACS. Strategies 3, 5, 7, and 9 are expectant management until the target gestational age of 36, 37, 38, or 39 weeks, respectively. Strategies 4, 6, and 8 involved expectant management until an amniocentesis test for fetal lung maturity at the indicated gestational age has been performed. In these strategies, the event that testing shows lung immaturity, amniocentesis is repeated weekly and cesarean delivery is performed either when lung maturity is confirmed or at 39 weeks of gestation, whichever comes first. Robinson et al concluded suggesting as preferred strategies in individuals with ultrasonographic evidence of placenta previa and placenta accreta delivery at 34 weeks of gestation. In women with risk of antepartum hemorrhage necessitating delivery between 1% and 7% at 34 weeks of gestation, then the preferred strategy suggested was to deliver at 37 weeks of gestation without performing amniocentesis. Only when the risk of serious hemorrhage necessitating delivery is less than 1%, a probability outside the range of published values, expectant management until 39 weeks of gestation became the most preferred strategy[21].The benefits of avoiding emergent delivery with earlier delivery must be weighed against the neonatal risks of iatrogenic prematurity. Although the absolute risk of death or long-term complications of prematurity with LPTB are extremely low, “softer” morbidities in these infants are common. McIntire DD et al noticed late preterm birth children have increased neonatal morbidity compared with birth at term. Respiratory distress, transient tachypnea, grades 1 or 2 intraventricular hemorrhage, sepsis work-ups, culture-proven sepsis, phototherapy for hyperbilirubinemia and intubation in the delivery room were significative more common in these infants[22]. Thus, decision-making for the timing of delivery across the LPTB, ETB and term births in women with stable placenta previa requires balancing the probability and severity of maternal hemorrhage at each gestational age versus the probability and severity of neonatal morbidity with delivery. The relationships between the probability of maternal hemorrhage increases and neonatal morbidity decreases with advancing gestational age[17]. In women with uncomplicated complete placenta previa seems reasonable to perform a cesarean delivery at 36-37 weeks of gestation, after the antenatal corticosteroids (ACS) administration at 35 weeks for the fetal lung maturity. Meanwhile in women with a placenta previa and additional co-morbidities (high body mass index, previous cesarean deliveries, episodes of vaginal bleeding) clinicians should considerer early preterm delivery.Once a decision to perform cesarean delivery has been made, questions arise about the anesthesiological management. In 1999 Frederiksen et al[23] conducted a 22 year analysis reviewing all women with placenta previa who underwent cesarean delivery in their center with the purpose to identify what was the safer anesthetic method in these women. Placenta previa was found in 514 women. The mean gestational age at delivery was 35.3 +/- 3.4 weeks. General anesthesia was used for delivery in 380 women and loco-regional anesthesia was used for 134 women. Prior cesarean delivery was a predictor of the need for hysterectomy. General anesthesia increased the estimated blood loss, was associated with a lower postoperative hemoglobin concentration, and increased the need for blood transfusion. Elective and emergent deliveries did not differ in estimated blood loss, in postoperative hemoglobin concentrations, or in the incidence of intraoperative and anesthesia complications regional and general anesthesia did not differ in the incidence of intraoperative and anesthesia complications. The authors concluded in women with placenta previa, general anesthesia increased intraoperative blood loss and the need for blood transfusion. Regional anesthesia appears to be safe[23].
4. Conclusions
- The debate regarding the optimal timing of delivery in women with complete placenta previa and, particularly, the management of the stable and asymptomatic complete placenta previa is still open. In the management of the present case on the basis of the following conclusions: 1) 36 weeks+0 were the best time to deliver in a patient with stable complete previa in the study of Zlatnik et al, but at 36 weeks the neonatal NICU admissions have a percentage of 22.1% and the need for mechanical ventilation 3%, compared to the respective percentage at 37 weeks, 11,8% and 1.1%. The neonatal mortality was similar in both groups (0,3% at 36 weeks and 0,2% at 37 weeks)[18];2) 38 weeks+0 were considered the best term to deliver in patient with stable previa and negative fetal lung maturity tests (PG or L/S ratio) at 37 weeks, but at 38 weeks the maternal morbidity is significantly higher than at 36 weeks (58.6% vs 15.0%)[18];3) absence of a previous cesarean section. A previous CS is significantly associated with risk of accretion.4) presence of a cervical length >30 mm;On the basis of the following point we decided to choose for an early term birth (ETB) at 37 weeks of gestational age rather than a late preterm birth (LPTB).
ACKNOWLEDGMENTS
- Valentina Pafumi has carried out English language editing for this article.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML