-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Obstetrics and Gynecology
2012; 1(3): 27-29
doi: 10.5923/j.rog.20120103.03
Misoprostol Sublingually Versus Vaginally for Labor Induction at Term: A Randomized Study
El Mehdi Hissane , Mohamed El Karroumi , Fawzi Mikou , Mahjoub Ghazli , Nourredine Matar
Department of Obstetrics and Gynecology “B”, Averoes Hospital, Casablanca, Morocco
Correspondence to: El Mehdi Hissane , Department of Obstetrics and Gynecology “B”, Averoes Hospital, Casablanca, Morocco.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Objective: To compare the efficacy of misoprostol 50 μg vaginally and 50 μg sublingually for labor induction at term. Materials and Methods: One hundred and twenty women were randomized to receive misoprostol 50 μg vaginally (n = 60) or 50 μg sublingually misoprostol (n = 60). The doses were given every 5 h (maximum 5 doses). Primary outcome measure was vaginal delivery rate. Induction to delivery time, delivery within 12 h, the number of misoprostol doses given and neonatal outcomes were secondary outcome measures. Results: Vaginal delivery rates were 75% in the sublingual group and 73% in the vaginal group (RR, 1.09; 95% CI, 0.4 - 2.4).The number of women delivering within 12 h was 20 (45.5%) in the vaginal group and 12 (27%) in the sublingual group (RR, 0.5; 95% CI, 0.3 – 1). There were no significant differences in the number of doses needed, incidence of contractility disturbances, or neonatal results. Conclusion: Sublingual misoprostol is as efficacious as vaginal misoprostol for induction of labor and neonatal outcomes are similar.
Keywords: Sublingual Misoprostol, Vaginal Misoprostol, Induction Of Labor, Effectiveness, Safety
1. Introduction
- The use of misoprostol for labor induction with a live fetus was first described in 1992, in the pioneering study by Margulies et al.[1]. Since then, decreasing doses have been proposed and different routes of administration of misoprostol have been tried effectively[2,3]. Recent studies have suggested the possibility of sublingual administration of misoprostol for labor induction[3-5]. However, more research is needed in this area. The administration of sublingual misoprostol is practical and simple. Women in fact prefer this route of administration than vaginal for comfort reasons. Our goal is to administer misoprostol to our patients exclusive sublingually as effective as vaginal. The present study was carried out to test the effectiveness and safety of 50 μg tablets of misoprostol sublingually every five hours for the induction of term labor, compared with the same dose administered vaginally.
2. Material and Methods
- A randomized clinical trial was conducted in Averoes hospital of Casablanca in Morocco between December 2009 and October 2010. The research ethic boards approved the study. All volunteers signed an informed consent form. Inclusion criteria were the following: singleton pregnancy, gestational age of 37 weeks or greater, live fetus, cephalic presentation, and Bishop score of 6 or less. Women were excluded in cases of fetal malformations, previous uterine scars, or any contraindication to vaginal delivery. By means of a computer-generated randomization table, 120 subjects were assigned in two groups, 60 women in each one, to receive repetitive doses of 50 μg of misoprostol (one quarter of a 200 μg tablet) by sublingual or vaginal route.The administration was repeated every 5 h until 3 or more uterine contractions of 40 s duration occurred over minutes, or when the maximum of 5 doses was reached. In the absence of active labor 5 h after administration of the last dose of misoprostol, failed induction was reported and cesarean section was performed. Fetal auscultation every 15 min was performed during labor in all patients, before, during, and after contractions. Cardiotocography was performed every 2 h or at shorter intervals at the discretion of the attending obstetrician. The uterine activity was clinically assessed every 30 min. The primary outcome measure was vaginal delivery rate. Secondary outcomes included the time from first misoprostol administration to initiation of labor and to delivery; duration of labor and number of misoprostol doses administered. Adverse effects included uterine contractility disturbances (uterine hyperstimulation), gastrointestinal symptoms such as nausea, vomiting, and diarrhea. Perinatal outcomes analyzed were fetal heart rate (FHR) changes during labor, intrapartum meconium passage, and Apgar scores at 5 min. We defined uterine hyperstimulation as uterine tachysystole (with five or more contractions in a 10 minute period for two consecutive 10 minute periods) or uterine hypertonus (a uterine contraction lasting for more than two minutes). The changes in fetal heart rate that we considered abnormal included persistent decelerations, fetal tachycardia (fetal heart rate > 160 beats per minute), fetal bradycardia (fetal heart rate < 120 beats per minute), or reduced short term variability (< 5 beats per minute). A total of 120 women were randomized. All women underwent the labor induction protocols at the participating hospital. There were no withdrawals or exclusions post randomization, and all women received the originally assigned treatment. Statistical analysis was performed using the statistical package SPSS. A significance level of 5% was adopted. Risk ratio (RR) and 95% confidence interval (CI 95%) were calculated to assess the magnitude of the association between outcomes and route of misoprostol administration.
3. Results
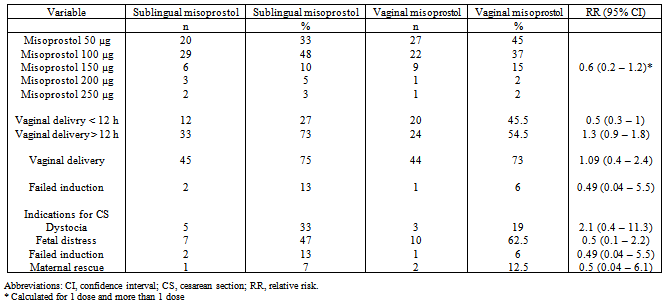
- There were no significant differences between groups regarding maternal age, parity, gestational age, or indication for the induction. Prolonged pregnancy (46% of participants in the sublingual group and 35% in the vaginal group, respectively) and hypertensive syndromes (35% in the sublingual group and 43% in the vaginal group) were the main indications for labor induction. Vaginal delivery was achieved in 75% participants in the sublingual group and in 73% in the vaginal group, but the difference did not reach statistical significance (RR, 1.09; 95% CI, 0.4—2.4) (Table 1).The number of misoprostol doses used was similar in both groups. About 33% of the women who received misoprostol sublingually and 45% of those who received misoprostol vaginally needed only 1 dose for initiation of labor. There were no significant differences between the 2 groups regarding number of deliveries achieved in fewer than 12 h, within 24 h, and after 24 hours , and failed labor induction (Table 1).The rates of contractility disturbances were similar in the 2 groups. The main indications for cesarean section were fetal distress and dystocia in both groups. Although the number of cesarean sections for fetal distress was almost greater in the vaginal group, the difference did not reach statistical significance (Table 1).
|
|
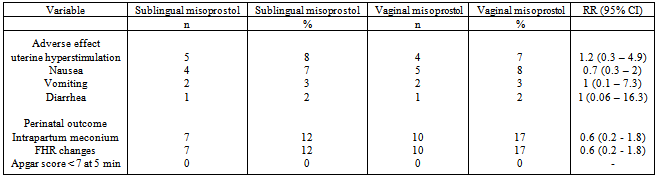
- Maternal adverse effects such as nausea, vomiting, and diarrhea were similar in the 2 groups.There were also no significant differences between the groups regarding meconium-stained amniotic fluid, FHR changes, or in Apgar scores less than 7 at 5 min (Table 2).
4. Discussion
- Because plasma levels of misoprostol are significantly greater when the same dose is administered sublingually rather than vaginally[6;12]. That expectation, however, was not confirmed by the findings, as the proportion of vaginal deliveries within 12 h of administration was slightly higher in the vaginal group. None of the other indicators of effectiveness were found to be better in the sublingual group. There was a 2% difference in the vaginal delivery rate in favor of the sublingual route, this difference did not reach statistical difference. Like in this study, studies performed by Moraes-Filho et al.[7] and Caliskan et al.[8] found no differences in rates of vaginal delivery, fetal distress, or successful induction between the vaginal and sublingual groups.The patterns of plasma levels achieved by the 2 routes suggest the possibility of a greater risk of hypercontractility with sublingual administration. This was not observed, on the contrary, the risk for cesarean sections due to fetal distress increased with the use of vaginal misoprostol. But the difference did not reach statistical significance (RR, 0.5; 95% CI, 0.1 2.2). Nevertheless, the apparent difference in numbers of cesarean section for fetal distress in this study is not consistent with a complete lack of difference in the incidence of hypercontractility, meconium staining of the amniotic fluid, and low Apgar score. Thus, the sublingual route can be considered as safe as the vaginal route for administration of misoprostol for the induction of labor. Failed labor inductions occurred in both groups (13% in the sublingual and 6% in the vaginal group) but without statistical significance. The failed induction rates were lower in the study by Moraes- Filho et al.[7], (10.3% and 4.9% for the sublingual and vaginal groups, respectively), but the authors continued misoprostol administration for 48 h, suggesting that prolonging treatment could improve the success rate.This trial adds to the substantial literature confirming that misoprostol is a highly effective pharmacologic agent for labor induction. As already suggested by other authors[5-7], the results tend to confirm that the sublingual route represents a valid alternative. According to 2 studies[9-11], the sublingual route appears to have the advantage of a greater acceptance by women than the vaginal route. It is understandable that it is more comfortable to place a tablet in the mouth than in the vagina.
5. Conclusions
- Thus, although in this study the sublingual administration of misoprostol 50 μg was neither more effective nor safer than the same dose administered vaginally, the limited sample size does not allow reaching definitive conclusions. Even if a small sample of our study preliminary results appear encouraging. To meet the convenience of our patients we will begin the same study with more patients and more spread duration. If the results are comparable to those in this study we can generalize the administration of misoprostol in our service for the induction of labour at term sublingual.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML