-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Research in Obstetrics and Gynecology
2012; 1(1): 1-14
doi: 10.5923/j.rog.20120101.01
Birth Weight in Relation to Fetal and Maternal Leptin and Insulin: A Systematic Review and Meta-analysis
Agzail S. A. Elhddad 1, Hany Lashen 1, 2
1Department of Human Metabolism, Academic Unit of Reproductive and Developmental Medicine, University of Sheffield, The Jessop Wing, Sheffield S10 2SF, UK
2Highest awarded academic degree: FRCOG
Correspondence to: Hany Lashen , Department of Human Metabolism, Academic Unit of Reproductive and Developmental Medicine, University of Sheffield, The Jessop Wing, Sheffield S10 2SF, UK.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
leptin and insulin have been implicated in fetal growth regulation and investigated repeatedly with controversial outcomes. A systematic review and meta-analysis were conducted according to MOOSE guidelines to establish if consistency exists in the reported literature so that the role of maternal and fetal insulin and leptin in fetal growth could be assessed. A robust recognised systematic methodology was used in the literature search and data extraction and analysis to avoid bias. SPSS version 16 and RevMan 4.2 were used for statistical analysis and the results presented as a Weighted Mean Difference (WMD) and 95% CI. Nineteen studies were included. Large for gestational age babies (LGA) had significantly higher cord serum insulin and leptin levels than was adequate for gestational age (AGA) ones[3.16 µIU/ml (0.85, 5.48) and -4.33 ng/ml (-5.30, -3.36) respectively]. Small for gestational age babies (SGA) had similar insulin levels but significantly lower cord leptin[21 µIU/ml (-5.69, 1.27)] than AGA babies[-3.07ng/ml (-4.57, -1.58)]. Maternal serum levels of leptin were similar between mothers of LGA, SGA and AGA babies. Conclusion: although both fetal insulin and leptin reflect the degree of fetal adiposity, leptin may have growth promoting properties.
Keywords: Leptin, Insulin, Fetal Growth, Systematic Review, Meta-Analysis
Article Outline
1. Introduction
- Fetal growth is a complex process that is influenced by multiple factors and has far-reaching health implications. Several hormones are believed to have a regulatory role. However, the relative contribution of many hormones remains speculative at best and usually controversial. The role of insulin and leptin among the complex network of factors controlling fetal growth is still a matter for debate. Insulin has long been known to augment fetal growth by stimulating nutrient transfer via its direct lipogenic effect, enhancing the bio-availability of IGFs[1-4] and was also found to up-regulate leptin secretion[5]. However, others believe that insulin has only a permissive role in fetal growth[6]. The role of leptin as an intrauterine growth modulator has been a subject of controversy. Healthy pregnant women have significantly higher serum leptin levels than healthy non-pregnant ones[7]. Maternal serum leptin increases progressively, peaking in the second trimester, plateauing towards term and returns to pre-gestational levels after delivery[8-9]. Leptin can be detected in cord serum as early as 19 weeks, increasing with advancing gestation[10]. Cord leptin concentrations were found to be directly correlated with birth weight and Ponderal index in several studies[11-13]. Higher circulating fetal and maternal leptin levels and the presence of leptin and leptin receptors in fetal and placental tissues[14] indicates that leptin is potentially a growth factor. However, the strong correlation between leptin and birth weight may merely reflect fetal adiposity and may not be related to fetal growth.No significant difference in cord leptin levels was found between small for gestational age (SGA) and appropriate for gestational age (AGA) babies[15] indicating that if leptin acts as a growth factor in-utero it has a weak non-regulatory role. Moreover, a more recent study reported no significant correlation between neonatal leptin and birth weight or birth BMI[16]. Several studies reported the absence of correlation between maternal serum leptin and birth weight[17-18].Accordingly, the roles insulin and leptin play in promoting fetal growth have been controversial mainly due to small sample size and clinical and methodological diversity in the published studies. A systematic review of the role of leptin and insulin in fetal growth in euglycaemic mothers and babies along the full growth spectrum was performed.
2. Objectives
- The objectives were to assess the extent of discrepancy or consistency in the available literature and to form an objective opinion based on the weight of evidence regarding the role of maternal and fetal leptin in relation to insulin in fetal growth.
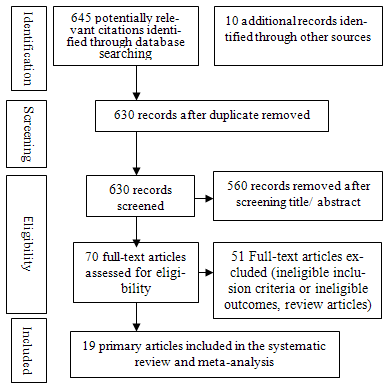 | Figure 1. Process of literature retrieval |
3. Materials and Methods
- The systematic review and meta-analysis were based on a detailed prospective protocol and was conducted according to MOOSE[19] guidelines.
3.1. Search Strategy, Study Selection, Study Quality Assessment and Data Extraction
- A comprehensive literature search of Medline (Pubmed and Ovid SP), Scopus, EMBASE and Web of Knowledge and Cochrane Library was performed for the original work conducted on humans between 1960 and March 2010. To obtain any articles missed by the electronic search and to minimize publication bias, scanning of the reference list of all related articles was performed together with search in the System for Information on Grey Literature in Europe to pick up the unpublished work. The UK Clinical Research Network portfolio database was used to search for relevant trials in progress. In case of a duplicate publication, the most recent or complete version was included. Searches were restricted to English language articles because of financial limitations. Advice for electronic search was obtained from an expert librarian at the Health Sciences Library, Royal Hallamshire Hospital in Sheffield, UK. Selected studies were eligible to be included if they met the following criteria: those reported on mothers with singleton pregnancies who were non-smokers, with no history of alcohol consumption or drug abuse during the pregnancy, free from any pregestational medical problems or pregnancy-induced complications that were likely to affect fetal growth. Studies conducted on the cord sera of term viable babies who were free from chromosomal or major structural anomalies were also eligible. Figure 1 illustrates the process of retrieval of the studies; selection of all the potential articles was carried out firstly by screening their titles and/or abstracts independently by two reviewers (A.E. and H.L.) according to pre-determined inclusion and exclusion criteria. The studies that compared the levels of maternal and/or cord serum insulin and leptin, individually or in combination, in relation to a specific pattern of fetal growth, irrespective of the study design, were included. The full manuscripts of all potentially appropriate abstracts were obtained and reviewed. The two investigators independently considered papers for eligibility and critically appraised the included studies according to criteria decided a priori to include only the studies of the highest quality. Any discrepancy was discussed by the two reviewers to reach a consensus. Data extraction was performed from the eligible studies using a pre-prepared proforma. The investigators were not blinded to the authors, institutions or journals in the retrieved studies.
3.2. Data Synthesis and Summary Of Study Results
- Table 1 demonstrates the individual study characteristics and the results of the included studies. Statistics were conducted using RevMan 4.2 (Copenhagen, the Nordic Cochrane Centre the Cochrane Collaboration, 2008) and SPSS version 16.0 (SPSS Inc., Chicago, Illinois, USA) and the data were presented as Weighted Mean Differences (WMDs) with 95% CI throughout the text. The primary analysis was the meta-analysis of WMDs and 95% CI of cord serum leptin and insulin levels for the studies comparing the babies whose growth was considered abnormal (study group) and AGA babies (reference group). Maternal insulin and leptin levels of the mothers who gave birth to babies with normal and abnormal growth were also analysed. The forest plot was firstly used to demonstrate the results of the individual study data and then the outcomes from the original studies were pooled using either a fixed effect model or random inverse variance effect model. A p value of < 0.05 was considered as a significant level.The in-between studiesheterogeneity was examined visually by inspecting the forest plots and then examined statistically using the Chi-square and I2 tests[20] and p value. A p value of < 0.1 and I2 > 50% or a Chi2 greater than its degree of freedom were considered statistically significant evidence of heterogeneity. Where there is statistical heterogeneity with the use of both fixed effect and the random inverse models, post hoc sensitivity tests were employed by exclusion of each study in turn and by subgroup analysis. Subgroup analyses according to the type of assay used and the background normal population were also performed where possible to investigate the potential impact of these confounding variables on the heterogeneity and statistical significance of the initial analysis.
|
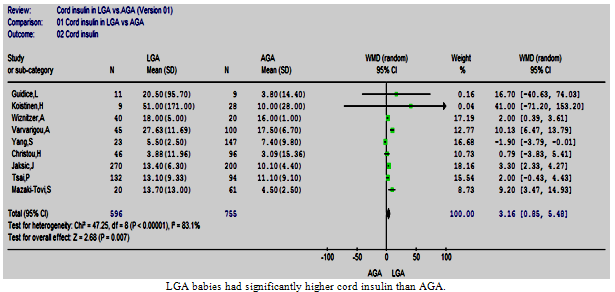 | Figure 2. Cord insulin in LGA vs. AGA |
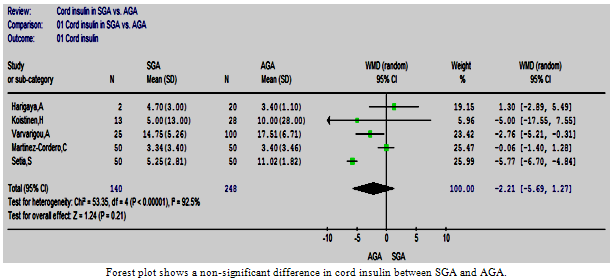 | Figure 3. The cord insulin in SGA vs. AGA |
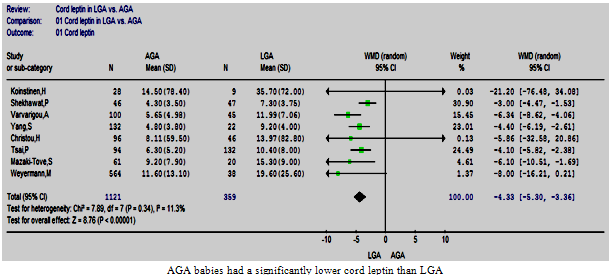 | Figure 4. Cord leptin in LGA vs. AGA |
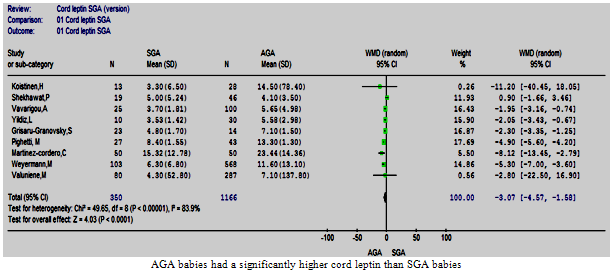 | Figure 5. Cord leptin in SGA vs. AGA |
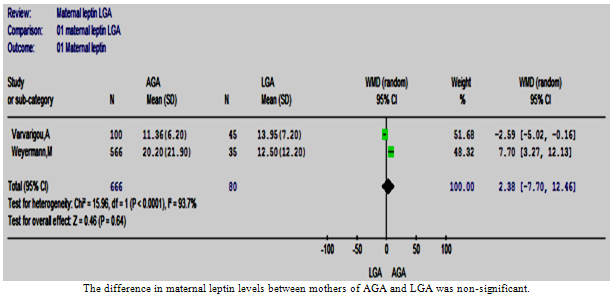 | Figure 6. The maternal leptin in LGA vs. AGA |
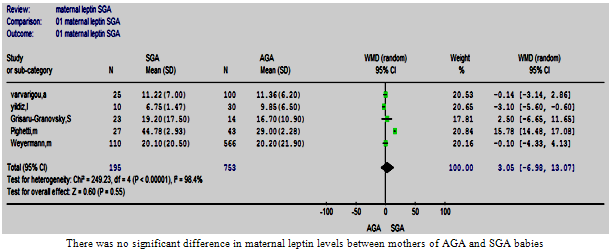 | Figure 7. Maternal leptin in SGA vs. AGA . |
4. Results
- Nineteen observational studies were eligible for inclusion in this systematic review and meta-analysis according to the predetermined protocol.
4.1. The Primary Analysis
4.1.1. Cord insulin in LGA vs. AGA
- Nine studies conducted on 1351 babies were eligible to be included in this review[22, 24-26, 28-30, 32, 39]. All these studies demonstrated either a significantly higher cord insulin level in LGA than AGA or no significant difference. In AGA babies, the mean (SD) cord insulin ranged between 3.09 µIU/ml (15.4) and 17.5 µIU/ml (6.7); however, the range of mean cord insulin was wider for LGA babies, from 3.88 µIU/ml (11.96) to 51 µIU/ml (171).The meta-analysis showed that cord insulin was significantly higher in LGA babies than AGA (p=0.007) and the pooled WMD (95%CI) was 3.16 µIU/ml (0.85, 5.48). The test of heterogeneity showed a statistically significant heterogeneity between these studies[Chi2 =47.25, df =8 (p <0.00001), I2 =83%] (Figure. 2). A post hoc analysis was performed and the homogeneity was achieved by the exclusion of two studies[22, 30]; however, the overall effect remained significant.
4.1.2. Cord insulin in SGA vs. AGA
- Five studies comparing the cord insulin level between 140 SGA and 248 AGA babies were retrieved and included in the review[27, 30, 32-34]. The results of these studies were controversial. The meta-analysis showed a non-significant difference in cord insulin levels between SGA and AGA babies (p=0.21) and the estimated pooled WMD (95%CI) was -2.21 (-5.69, 1.27). There was however a statistically significant heterogeneity between these studies (Figure. 3), and homogeneity was achieved by exclusion of one study[27] and the difference remained non-significant.
4.1.3. Cord leptin in LGA vs. AGA
- Eight studies comparing the cord leptin levels between 359 LGA and 1121 AGA babies were included in this review[26, 28-30, 32, 36-38]. The included studies were homogeneous[Chi2 =7.89, df =7 (p=0.34), I2 = 11.3%], despite the use of different assays and varied background of the included populations. The meta-analysis confirmed that the LGA babies had significantly higher cord leptin levels than AGA peers, the estimated pooled WMD (95%CI) was -4.33 (-5.30, -3.36) p<0.00001 (Figure. 4).
4.1.4. Cord leptin in SGA vs. AGA
- Nine studies that compared the cord leptin of 350 SGA and 1166 AGA babies were analysed[15, 23, 30-32, 34-36, 38]. The mean and standard deviation of cord leptin concentration in the AGA groups ranged between 4.1 ng/ml (3.5) and 23.44 ng/ml (14.36), whereas that for SGA ranged from 3.3 ng/ml (6.5) to 15.32 ng/ml (12.78). The overall difference was significant with the higher cord leptin levels in AGA than SGA babies, p<0.0001 and the pooled WMD was -3.07 (-4.57, -1.58) (Figure. 5). There was evidence of heterogeneity between the studies[Chi2 =49.65, df =8 (p<0.00001), I2 = 83.9%], homogeneity was achieved by excluding two studies[23, 36] without changing the result of the initial analysis.
4.1.5. Maternal leptin in LGA vs. AGA
- Herein, only two studies were included[30, 36]. The meta-analysis of these studies showed a statistically non-significant difference between the two groups of mothers[WMD was 2.38 (-7.70, 12.46), p value = 0.64]. The test of heterogeneity was statistically significant[Chi2 =15.96, df =1 (p<0.0001), I2 = 93.7%] (Figure. 6). However, it was not possible to conduct sensitivity analysis due to the small number of studies included.
4.1.6. Maternal leptin in SGA vs. AGA
- Five studies were eligible and meta-analysed[15, 23, 30, 35-36]. One study reported that the mothers of SGA babies had higher circulating leptin levels than those who delivered AGA babies[23]. Another study reported exactly the opposite[35]. A non-significant difference was found between the two groups of mothers in the remaining three studies. A verdict that was upheld by the meta-analysis, and the pooled WMD (95%CI) was 3.05 (-6.98, 13.07) (p=0.55). These studies were heterogeneous (Figure. 7). Homogeneity was achieved (Chi2 =3.51, df =3 (p <0.32), I2 = 14.6%) by excluding the study that demonstrated a significantly higher WMD for maternal leptin in mothers of SGA than AGA babies[23]. The estimated pooled WMD remained non-significant.
4.1.7. Maternal insulin in LGA vs. AGA
- Only one comparative study[39] was eligible to be included in this systematic review. This study reported a non-significant difference in maternal serum insulin between mothers of LGA and AGA babies; the mean (SD) was 28µIU/ml ±7 and 31µIU/ml ± 5 respectively.
4.2. Subgroup Analysis
- The included studies used different assay methods to determine insulin and leptin levels and were conducted on populations of different ethnic backgrounds. There was statistically significant heterogeneity between the studies in almost all of the meta-analyses. The assay method and the population background can potentially influence reproducibility of studies and bring about the observed heterogeneity[40-42]. However, the studies included in the comparison of cord leptin between LGA and AGA babies were homogenous despite the use of different assay methods and diversity of the population background. Therefore, other methodological factors are likely to have been a source of heterogeneity. The potential effects of the assay method and the reference population on in-between studies observed heterogeneity was examined in most of the conducted analyses.
4.2.1. Subgroup analysis according to the assay method: two assay methods were used in the included studies; radioimmune assay (RIA) and immune radiometric assay (IRMA)
4.2.1.1. Cord insulin in LGA vs. AGA
- Five and two studies using RIA and IRMA respectively were meta-analysed in two different subgroups. The overall effect in the RIA group showed a significantly higher cord insulin levels in LGA compared to controls, WMD= 4.78 µIU/ml (1.52, 8.04), (p=0.004) (Figure. 8.1) which is in agreement with the full-group analysis. However the test of heterogeneity remained significant. The studies using a commercial IRMA were homogeneous, but the difference in circulating cord insulin between LGA and AGA babies became non-significant (Figure. 8.2). It was not possible to do subgroup analysis for the other two studies as they used different assays.
4.2.1.2. Cord insulin in SGA vs. AGA
- The RIA method was used in four studies. The subgroup analysis of these studies also suffered from statistically significant heterogeneity; however, the result of the primary analysis did not change (Figure 8.3).
4.2.1.3. Cord leptin in SGA vs. AGA
- Two subgroup analyses were possible; the RIA subgroup that contained five studies and IRMA subgroup that contained three studies (Figures 8:4, 5). The WMD remained significant in both subgroup analyses. However, a statistically significant heterogeneity was encountered in the IRMA but not in the RIA subgroup.
4.2.1.4. Maternal leptin in SGA vs. AGA
- Two separate subgroup analyses were conducted according to the assay method (IRMA and RIA). Both analyses supported the result of the primary analysis; nonetheless, the test of heterogeneity remained significant (Figures 8:6, 7).
4.2.2. Subgroup Analysis According to The Reference Population
- In order to assess the effect of ethnicity and the natural differences in population on the results of the studies, the mean and 95% CI of the cord insulin levels of the AGA babies were plotted (Figure. 9) and one way ANOVA test was used to find out the studies with similar levels. A meta-analysis was carried out that included only the studies that had similar reference AGA population. This was repeated for the cord and maternal leptin. Accordingly, homogeneity was achievable in every analysis and the overall effect remained in agreement with the primary analyses in most of the analyses (Figures 10: 1- 3).
5. Discussion
- The mechanisms of fetal growth and the factors regulating fetal growth are not certain yet. There are several factors that have been investigated repeatedly with controversial outcomes. Insulin and leptin are among these factors. Insulin is essentially a growth factor with strong anabolic properties that also influence the availability of nutrients to the fetus and the bioavailability of other growth factors[1, 43-44].The role of leptin in fetal growth is not clear and has been a subject of debate. Several studies reported a positive correlation between insulin and leptin and many of the babies’ anthropometric indices at birth[28, 31, 45-46]. Others did not report such a relationship[17, 34, 36, 47]. However, identifying an association between insulin or leptin levels in the mothers or their babies and the babies’ anthropometric indices does not prove the role of either in all aspects of the growth process. Further, it does not indicate whether such a relationship is linear across the entire growth spectrum or more preferentially evident at one end. Furthermore, a strong correlation is likely to exist between the fetus’s adiposity, rather than linear growth, and both insulin and leptin levels which, if correct, should be evident at any point of the growth spectrum but much more likely in LGA babies. To investigate this point several meta-analyses of the comparative studies of the babies across the fetal growth spectrum i.e. AGA, SGA and LGA were conducted. The objective was to establish if a linear relationship existed between birth weight as a measure of fetal growth and insulin and leptin, and whether this relationship was consistent at both ends of the growth scale. As it is difficult to meta-analyse correlation studies, it was hypothesised that if the trend of evidence from the studies comparing leptin and insulin between SGA or LGA and AGA babies consistently shows a linear relationship throughout the growth spectrum, this would plausibly support a role for insulin and leptin in promoting or limiting fetal growth. Post hoc analysisFigures: 8.1- 8.7: Subgroup analyses according to the assay used
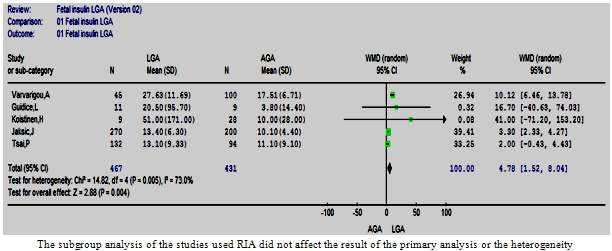 | Figure 8.1. The cord insulin in LGA vs. AGA in studies used RIA |
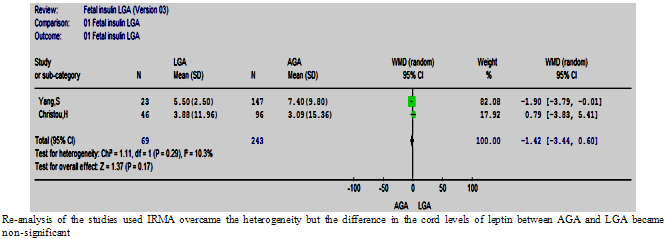 | Figure 8.2. The cord insulin in LGA vs. AGA in studies used IRMA |
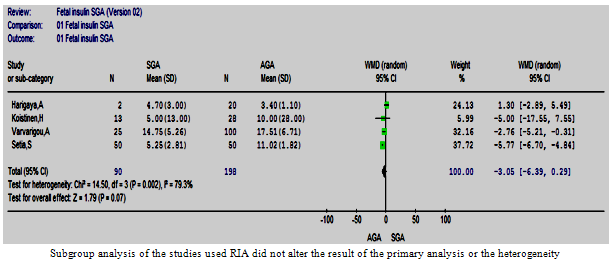 | Figure 8.3. Cord insulin in SGA vs. AGA in studies used RIA |
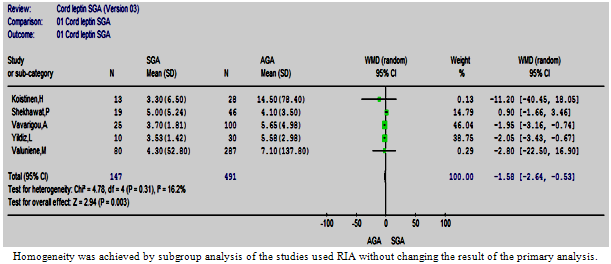 | Figure 8.4. The cord leptin in SGA vs. AGA in studies used RIA |
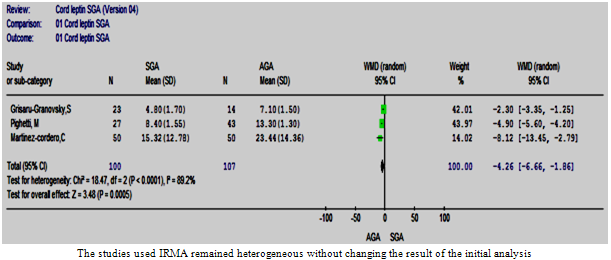 | Figure 8.5. The cord leptin in SGA vs. AGA in studies used IRMA |
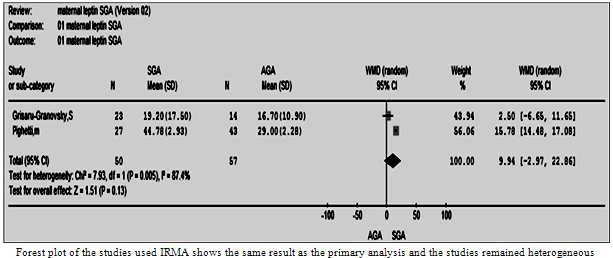 | Figure 8.6. The maternal leptin in SGS vs. AGA using IRMA |
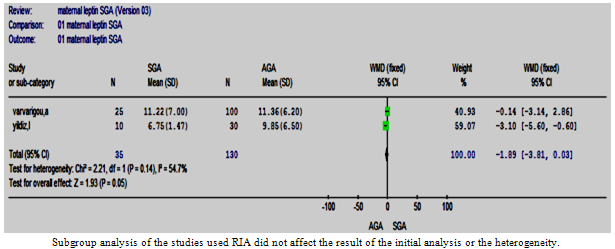 | Figure 8.7. The maternal leptin in SGS vs. AGA in studies used RIA |
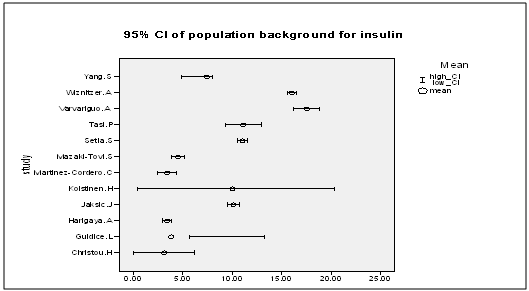 | Figure 9. The mean and 95% CI of cord insulin for the normal background population |
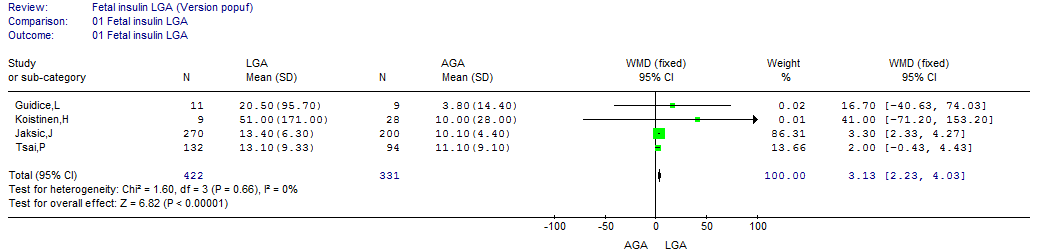 | Figure 10.1. The cord insulin in LGA vs. AGA according to background population |
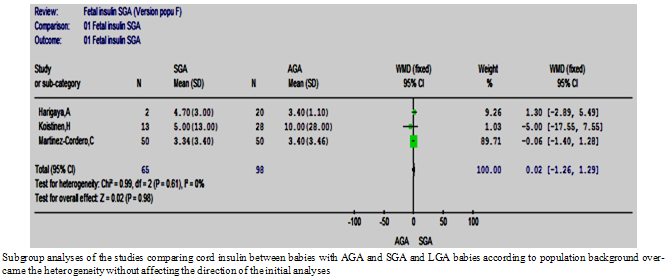 | Figure 10.2. The cord insulin SGA vs. AGA according to background population |
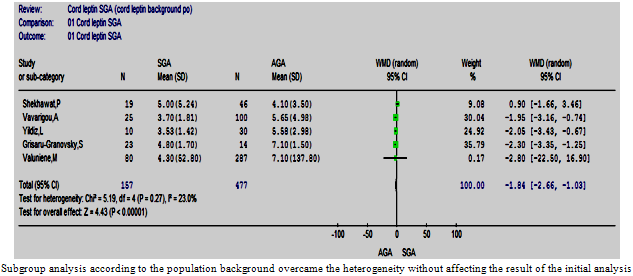 | Figure 10.3. The cord leptin in SGA vs. AGA according to background population |
5.1. Summary of the findings
5.1.1. Leptin
- Eight studies comparing cord leptin between LGA and AGA babies were homogeneous despite the use of different assays and the population diversity. The meta-analysis confirmed significantly higher cord leptin levels in LGA than AGA babies. At the other end of the growth spectrum, the meta-analysis of nine studies showed a significantly lower cord leptin in SGA compared to AGA babies and this was previously reported[48]. This proves a linear relationship between fetal growth and/or adiposity and fetal leptin levels. The meta-analyses showed no significant difference between mothers of AGA babies and those with LGA or SGA. However, it was difficult to be categorical about any relationship between maternal leptin and fetal birth weight or fetal adiposity because of the small number of included studies.
5.1.2. Insulin
- Insulin has a strong adipogenic effect, as evident from the studies in mothers with gestational diabetes, leading to macrosomia[49]. However, some studies have denied that fetal insulin is correlated with birth weight in non-diabetic mothers[34, 50]. This meta-analysis showed higher insulin levels in the cord blood of LGA than AGA babies and no difference at the other end of the growth scale. This denies a linear relationship between insulin and fetal growth suggesting that the role insulin plays in fetal growth is primarily to signal nutrient availability and its levels correlate with the size of the transplacental nutrient transfer[44], thus aiding fetal adiposity rather than linear growth. Therefore, it is unlikely that insulin plays a significant role, if any, in the pathogenesis of IUGR. Piecing the findings of these meta-analyses together, LGA babies had statistically significant higher cord serum insulin and leptin levels than AGA babies while SGA babies had significantly lower cord leptin but similar insulin levels compared to AGA ones. Accordingly, the lack of linearity in the relationship between insulin and fetal birth weight and the presence of such linearity between leptin and birth weight throughout the birth weight scale suggests that both insulin and leptin have different roles to play in fetal growth. Besides being a marker of mature adipocytes, leptin may influence linear growth more than insulin. It should be mentioned that a relationship does exist between leptin and insulin levels[26, 30, 32, 46] that is explained on the grounds that insulin stimulates leptin secretion[5]. However, given the findings of this review it is likely that the relationship between insulin and leptin in LGA babies is fortuitous rather than functional.
5.2. Heterogeneity
- The presence of heterogeneity in some of these meta-analyses may reduce the impact of the findings. The observed heterogeneity can be explained by the presence of clinical and methodological as well as populations’ diversity in the included studies. However, it has been established that the encountered heterogeneity was likely to be due to natural population differences. The difference in insulin resistance between different ethnic groups has been reported during intrauterine life as well as in adults[51-52]. In support of this, the difference in cord insulin and c-peptide has been reported among different ethnic groups[41-42]. Furthermore, cord insulin levels have been reported to be affected by the mode of delivery[53] which adds another source of heterogeneity. The difference in sensitivity of used assays is a likely source of heterogeneity; nonetheless, the subgroup analyses according to the assay technique yielded inconsistent results, thus weakening its influence as a source of heterogeneity. However, the presence of heterogeneity was not invariable as evident from the studies that compared the cord leptin between LGA and AGA babies despite the used of different assay methods. The definition which is likely to vary in the SGA group is another source of heterogeneity that can be difficult to overcome.
5.3. Strengths and Limitations
- The strength of this review lies in the comprehensive literature search, and the strict inclusion and exclusion criteria to ensure the quality of the studies included in the review. All comparative studies were searched without applying study design restriction in literature retrieval, thus allowing capture of almost all related studies. The review protocol was developed before the start of the study to minimize selection bias. To minimize reviewer bias study selection, data extraction and the quality of each study was assessed independently by the two reviewers and in cases of disagreements consensus was reached through panel discussion between the reviewers. Although only the studies that stated clearly the pattern of fetal growth were included, different studies gave different thresholds for definitions of SGA and LGA. Another problem was that some studies did not specify clearly if the low birth weight babies were normal constitutionally small babies or suffered from IUGR. Leptin and insulin secretions follow a circadian rhythm[54-55]. The included studies collected the blood samples from non-fasting participants at all times during the day and this could also represent a source of in-between studies heterogeneity and should be considered in future research.
5.4. Guidelines for Future Research
- Standardized threshold of different growth patterns need to be used in future trails to be able to compare outcomes of different studies. In future similar research projects, all possible fetal anthropometric measures should be taken into account, as a baby’s birth weight alone lacks enough sensitivity to address the roles of the different growth factors, especially with regard to linear growth.
6. Conclusions
- Fetal insulin and leptin play different roles in fetal growth at either end of the growth spectrum. Although both reflect the degree of adiposity, leptin is likely to have potential growth factor properties that may influence linear growth. Based on our findings, maternal leptin seems to play an insignificant role in fetal growth regulation. LGA babies tend to have higher insulin that is likely to be due to higher levels of nutrient transfer. Further studies are needed to confirm whether maternal insulin is also implicated. Any association found in this review may inform the design of future studies.
ACKNOWLEDGEMENTS
- The authors acknowledge Ms Frances M Ludlow, an expert Librarian at the Health Science Library, Royal Hallamshire Hospital in Sheffield, UK for her advice on electronic searches.
Conflict of Interest
- The authors have nothing to disclose.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML