Théophile Razafitsiferana, Hervéa Kall
University of Antsiranana, Faculty of Sciences, Chemistry Department, Mineral Chemistry and Environment Program
Correspondence to: Théophile Razafitsiferana, University of Antsiranana, Faculty of Sciences, Chemistry Department, Mineral Chemistry and Environment Program.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Background: Growing demand for sustainable materials is driving research into biobased composites. These materials combine binders of natural origin, such as polysaccharides (starch, alginate, chitosan...), and reinforcements derived from carbonaceous waste, such as plant charcoal powder.The main objectives are to- Reduce the use of petroleum-based plastics.- Recycle agricultural and industrial waste. - Develop biodegradable materials with good mechanical and thermal properties. Study objectives: - Formulate composites by combining a polysaccharide binder and a charcoal powder filler. - Characterize the composites' physicochemical and mechanical properties. - Study the chemical interaction between binder and reinforcement. - Assess biodegradability and potential uses (packaging, insulation materials, etc.). Methods: a. Preparation of composites - Extraction or commercial use of polysaccharides (starch, chitosan...). - Drying and grinding of vegetable charcoal to obtain a fine powder. - Mixing in various proportions (e.g. 10%, 30%, 50% powder). - Moulding or hot-pressing to shape. b. Chemical and physical analysis - analysis of chemical bonds and interactions between binder and coal powder. - (X-ray diffraction): study of material crystallinity. - thermal analysis (stability, degradation point). - observation. Resultats: Polysaccharide extraction yield by source: - Dioscorea sp (1700 g); Extracted polysaccharide: Starch; Obtained mass: 133 g; Yield: 7.8%. - Scylla serrata (50 g shell); Extracted polysaccharide: Chitosan; Obtained mass: 4.08 g; Yield: 8.16%. - Cassia hypophallus (20 g seeds); Extracted polysaccharide: Galactomannan; Obtained mass: 4 g; Yield: 20%. - Hydroclathrus clathrus (54 g seeds); Extracted polysaccharide: Aliginate; Obtained mass: 1.16 g; Yield: 2.14%. Details on the chitosan extraction process: Initial quantity of shell powder: 50 g, - After deproteinization: 66% (light gray powder) - After demineralization: 10.5% (off-white powder) - After deacetylation: 8.16% (off-white powder). Details of the galactomannan extraction process: - Quantity of seed powder: 20 g (brown in color) - Yield after precipitation: 20% (camel hair-colored powder). Results of combustion tests for polysaccharide briquettes (mass of 19 g each): - Starch; Burning time: 85 minutes; Observations: Initial odor, no smoke. - Chitosan; Burning time: 83 minutes; Observations: Initial pleasant odor, presence of smoke. - Galactomannan; Burning time: 53 minutes; Observations: Presence of smoke from the beginning. - Alginate; Burning time: 133 minutes; Observations: Presence of odor at the beginning of combustion, no smoke. Conclusions: - Formulated composites offer a good compromise between durability and eco-compatibility. - Plant charcoal, as a reinforcement, improves thermal stability and mechanical properties while maintaining biodegradability. - This type of material can be considered for non-structural applications, such as packaging, thermal insulation, or as eco-craft materials. Combustion of the starch-bound composite is similar to that of the galactomannan-bound composite, but its extraction yield is low. It would therefore be necessary to harvest Dioscorea sensibarensis during its vegetative resting period in order to gain a better understanding of its starch content, although its use remains conceivable for the preparation of our biosourced composite.
Keywords:
Biosourced, Composite, Combustion charbon powder
Cite this paper: Théophile Razafitsiferana, Hervéa Kall, Chemical Study of Bio-Source Compositions "Binder: Polysaccharide and Reinforcement: Charcoal Powder", Resources and Environment, Vol. 15 No. 1, 2025, pp. 11-20. doi: 10.5923/j.re.20251501.03.
1. Introduction
Energy demand is a fundamental need for every family, as all households use it, particularly for cooking. In modern countries, households rely on electricity, natural gas, and microwaves to meet their domestic needs.In Madagascar, whether living in urban or rural areas, households primarily rely on firewood, charcoal, and dried zebu dung as energy sources. However, this almost exclusive reliance on wood energy has led to significant deforestation on the island, with environmental consequences already felt by current generations.In this context, the development of biogas from agricultural organic waste, straw, and animal manure appears to be a promising alternative. Particular attention is also being paid to the production of fuel briquettes from household waste. To support these advances, a research topic has been selected: "Chemical study of bio-based composites, binder: polysaccharide, reinforcement: carbon powder."This study is part of the development of briquette production, with the aim of providing biological binders adapted to the requirements of fuel briquette manufacturing technology.
2. Bibliographic Summary
Activated carbonActivated carbon is an adsorbent material made from carbon-rich raw materials, such as wood. In principle, any organic matter containing carbon can be used to produce activated carbon.Raw materialsThese come from a wide variety of carbon-containing sources, of plant, animal, or mineral origin.• Plant origin:o Inedible agricultural waste, such as fruit pits, coconut shells, or sugarcane bagasse.o Cereal straw and hulls, such as wheat and rice. o Wood in the form of shavings or sawdust, such as birch, oak, eucalyptus, or lignite.• Animal origin:Activated carbon can be obtained from animal bones, but also from their blood, or even their flesh.• Mineral origin:Mainly derived from combustible materials such as coal, coke, or peat. It can also come from the recovery of residues from the oil industry or the pyrolysis of activated sludge.Types of CoalDifferent Types of CoalThere are two main categories of coal: natural coal and charcoal.Natural CoalsThe generic term "coal" covers several types of fossil fuels whose level of transformation (or maturity) and calorific value vary considerably. There are four main types:• Peat• Lignite• Subbituminous coals• AnthraciteCharcoalAs its name suggests, charcoal is produced by carbonizing wood. Its production can be carried out using different processes used throughout the world: the traditional method, the improved traditional method, and the modern method using metal furnaces.In Madagascar, the traditional method is the most commonly used.PolymersA polymer is a material made up of macromolecules, organic or inorganic, formed by the repetition of a basic unit called a "monomer." • Monomer: A simple molecule capable of reacting with other monomers to form a polymer. Unlike a polymer, a monomer has a low molecular weight.• Polymerization: A chemical reaction during which monomers join together to form high-molecular-weight polymers.BindersBinders are substances, natural or synthetic, of mineral or organic origin, used to ensure cohesion between different materials.Classification of binders:• Mineral binders: Finely ground powders obtained by processing rocks.• Organic binders: Natural or synthetic resins used to bind fillers and fibers in composite materials.• Natural polymersStarch is the main source of carbohydrates in plants. It is found in large quantities in roots and tubers (25 to 50%), as well as in seeds (25 to 50%). • Chemical structure of starch: it is a polysaccharide composed of two polymers: amylose and amylopectin. Starch appears in the form of grains that can be observed under a microscope.However, there are few α-(1-6) linkages. Amylopectin, on the other hand, is a macromolecule formed by the association of glucopyranose residues mainly linked by α (1-4) and α (1-6) bonds, which give it its branched structure. These are shown in the figure below.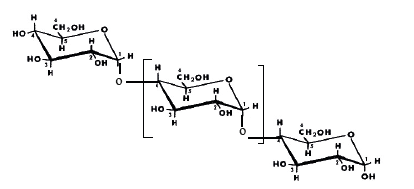 | Figure 1. Simplified diagram of the chemical structure of amylose according to Buléon [1] [5], [7] |
• The proportion of amylose varies between 20 and 30% depending on the botanical origin of the starch. Amylopectin, on the other hand, constitutes the majority fraction of most starches. The exact composition of amylose and amylopectin depends on the original plant source.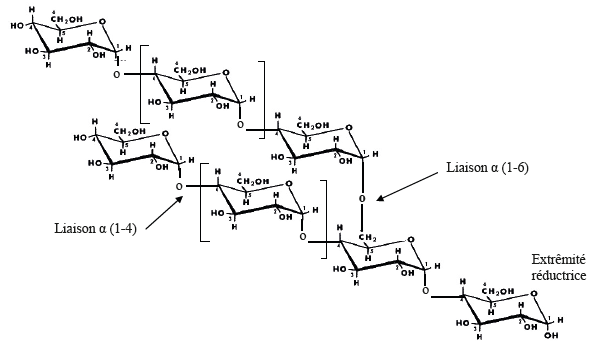 | Figure 2. Simplified diagram of the chemical structure of amylopectin according to Buléon |
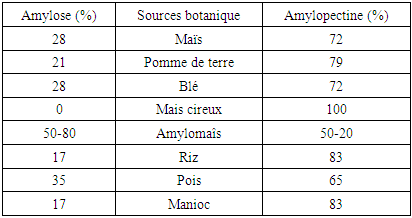
Table 1. Amylose and amylopectin contents according to different starch sources. [3] [4]
 |
| |
|
ChitosanChitosan is a polysaccharide composed of copolymers of glucosamine and N-acetylglucosamine. It is obtained by the deacetylation of chitin, extracted primarily from crustacean shells.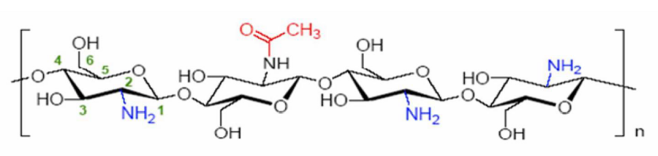 | Figure 3. Chemical structure of chitosan [2], [6] |
AlginateAlginate, or alginic acid, is a natural polysaccharide produced by certain brown algae and some bacteria. It is a polyuronide composed of D-mannuronic acid (M) and L-guluronic acid (G).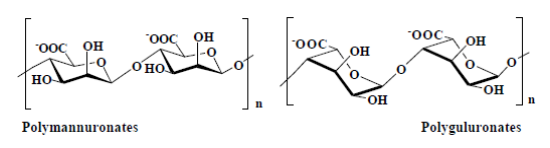 | Figure 4. Structure of alginates |
GalactomannansGalactomannans are used in the food industry as stabilizing agents. They serve to thicken preparations (sauces, drinks), stabilize suspensions and emulsions (such as chocolate milks), retain water and delay crystallization (in deli meat powders or ice creams), and form gels (for example, in jams).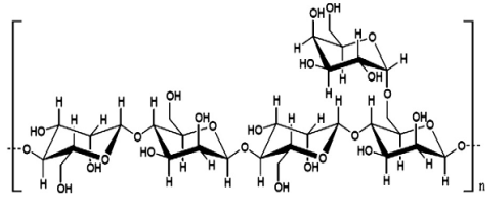 | Figure 5. Functional properties of galactomannans [8] [9] [10] |
3. Materials and Methods
Extraction and Production of Native Starch PowdersMaterials Used• pH paper is used to determine whether the solution is acidic, basic, or neutral, while wearing gloves protects against toxic substances.• Dioscorea Sansibarensis | Figure 6 |
Classification (Burkill & Bathie, 1950) [11] [12]Kingdom: PlantsPhylum: ANGIOSPERMAESubphylum: SPERMATOPHYTESClass: ANGIOSPERMOPSIDASubclass: LILIIDAE LILIIDAEOrder: DISCOREALESFamily: DIOSCOREACEAEGenus: DioscoreaSpecies: sansibarensisScientific name: Dioscorea sansibarensis Pax.Common name: “BabangaThis tuber, rich in starch, presents a high toxicity in its aerial part, in particular during the resumption of vegetation. It must therefore be the subject of a specific preparation in order to eliminate this toxicity before any use.ProcedureThe extraction of the various starches was carried out following the protocol described by Willinger (1964), modified by Alves, Grossmann, and Silva (1999).Peeling and WashingIn the laboratory, the samples were carefully washed with tap water, sorted, peeled with a stainless steel knife, and then rinsed three times with distilled water to remove any dirt or impurities.SoakingThe Dioscorea sansibarensis tubers were first cut into cylinders approximately 10 cm long and then macerated in a basin for 72 hours to eliminate hydrocyanic acid, a highly toxic substance. The pH of the solution was measured every 24 hours using a pH meter. Grating and SievingThe materials were then grated using a grater to obtain a paste consisting of fine fragments. These fragments were then mixed in equal proportions with distilled water and macerated using a spatula. The mixture was filtered through a white cotton cloth with a mesh size of approximately 200 μm. This process was repeated several times, until the filtered liquid became clear (approximately four times). The resulting liquids together constitute the starch milk.Decanting and DryingThe mixture was first allowed to stand for 4 hours at 4°C to allow sedimentation. The resulting deposit was then washed four times with distilled water and placed in a water bath at 50°C for 24 hours. Once dried, the starch is ground using a mill and sieved to obtain particles of a size less than or equal to 50μm. This product constitutes native starch with fine particle size. | Figure 7. Soaking Dioscorea slices |
 | Figure 8. Dioscorea paste |
 | Figure 9. Starch milk [14] |
 | Figure 10. Dried starch [13] |
Extraction and Production of Chitosan PowdersMaterials, Reagents, and Solvents UsedProtective gloves were used to handle the toxic products. Crab shells (Scylla serrata, Malacostraceae family) were ground using a grinder, and the resulting powder was sieved through fine-mesh sieves. The mass of the samples was determined using an electronic balance. Hazardous substances were stored in aluminum foil.A flask was used to prepare the solutions. A magnetic stirrer was used to homogenize them. The pH of the solutions was measured using pH paper. The solid and liquid phases were separated using filter paper.The mixtures were heated in a water bath, while the residues were dried in an oven. Distilled water was used as the standard solvent and for rinsing the equipment. Deproteinization was performed with a potassium hydroxide solution, while demineralization was carried out using a hydrochloric acid solution. Finally, the filtrate was precipitated by the addition of ethanol.ProcedureAfter collection, the shells were dried in an oven at 50°C for 72 hours. Once dry, they were ground using a mesh grinder (Philips Mini Blender), then sieved using sieves with mesh sizes of approximately 800 μm and 1000 μm. | Figure 11. (A) Scylla serrata shells, (B) Ground material sieved through a 1000 μm sieve, (C) [15] [16] [17] |
PREPARATION OF A POLYSACCHARIDE-BASED BINDERTo ensure good cohesion between the coal particles and the strength of the fuel briquettes, the addition of a binder is possible. Commonly used binders include starch, gum arabic, molasses, and clay. For this study, we selected starch, cassia gum, a polysaccharide extracted from brown algae, and chitosan as binding agents.• Starch-based binderMaterials usedGloves are used to protect against toxic substances. An electronic balance is used to measure the mass of the products. Aluminum foil is used to contain hazardous substances. Boiling temperature is reached using a hot plate. The amount of water is measured with a test tube. A flask is used to prepare the starch solution, while a pot was used to prepare the binder. The mixtures were stirred with a spoon. The final product was stored in a tightly sealed jar, then placed in the refrigerator.Products used and solvents: Dioscorea starch, tap waterProcedureSixty grams of starch were dissolved in a little tap water at room temperature to obtain a starch milk. At the same time, 500 mL of water was brought to a boil on a hot plate. As soon as the mixture began to boil, the prepared starch milk was poured into the pot containing the boiling water, stirring constantly, until a viscous liquid was obtained. This mixture was then cooled to room temperature before being stored for preservation. | Figure 12. Starch glue [18] |
• Chitosan-based binderMaterials and reagents usedThe mixture was prepared in a glass jar, using a magnetic stirrer during agitation.• The acetic acid solution was used to reduce viscosity, while glycerol was used as a humectant. Distilled water was used to prepare the solution.• Substrate: Chitosan powder.Procedure:A mixture containing 1% acetic acid and 2% glycerol was prepared, then 2 g of chitosan was dissolved in this solution with stirring at room temperature, resulting in a chitosan glue. | Figure 13. Chitosan glue [19] |
• Cassia gum-based binderMaterials and reagents usedA beaker was used to dissolve the polysaccharide powder, stirring with a magnetic stirrer.• An HCl solution was used to reduce the viscosity, with distilled water serving as the solvent.• Substrate: Galactomannan or Cassia gum.ProcedureTo do this, the gum was dissolved in distilled water by adding a few milliliters of HCl solution while stirring with a magnetic stirrer, until a paste with slightly reduced viscosity was obtained.A beaker was used to solubilize the polysaccharide powder, stirring with a magnetic stirrer.• An HCl solution was used to reduce the viscosity, with distilled water serving as the solvent.• Substrate: Galactomannan or Cassia gum.ProcedureTo do this, the gum was solubilized in distilled water by adding a few milliliters of HCl solution while stirring with a magnetic stirrer, until a paste with slightly reduced viscosity was obtained.Two different samples were selected:First sample: 2 g of viscous gum were solubilized in 1.5 mL of distilled water and 1 mL of 0.1 N HCl.Second sample: For 2 g of dry gum, 9 mL of 0.1 N HCl and 7 mL of distilled water were required. Solubilization was achieved by gradually adding distilled water and hydrochloric acid until the desired viscosity was obtained.Alginate-based binderMaterials and reagents used• A beaker was used to dissolve the alginate powder, with stirring performed using a magnetic stirrer.• Distilled water was chosen as the solvent.• Substrate: dried alginate powder.ProcedureThe preparation consisted of a simple mixture of distilled water and alginate powder, with stirring until the desired consistency was obtained.In this operation, 1.16 g of alginate was dissolved in 3 mL of distilled water with stirring.POLYSACCHARIDE GELLATION TESTMaterials and Reagents UsedThe required quantities of each polysaccharide and reagents were measured using a precision balance. 16 mL of distilled water were used to prepare the solutions. Stirring to facilitate dissolution was performed using a magnetic stirrer. A water bath was used for heating, and freezing was performed in a refrigerator at 4°C. In principle, the gelation of each polysaccharide x is induced by different metal cations (gelation promoters): Na+ and K+.Two saline solutions were used in this study:• 1M potassium chloride (KCl)• 1M sodium chloride (NaCl)Experimental Procedure:Solutions of 1M potassium chloride (KCl) and sodium chloride (NaCl) were prepared. In each tube, 0.1 g of dry polysaccharide extract is dissolved in 4 mL of 1 M saline solution. The mixture is then stirred with a magnetic stirrer for 5 hours at 60°C. After stirring, the behavior of the mixture is observed to determine the physicochemical properties of the polysaccharide. Each sample is then stored in a refrigerator at 4°C overnight, before being left at room temperature for a few minutes to evaluate the characteristics of each sample.PREPARATION OF BIO-SOURCED COMPOSITESTo ensure adhesion between the charcoal particles and the strength of the composites, a binder was added. For our study, we selected four types of binders: starch, chitosan, alginate, and cassia gum. Combining these binders with charcoal powder produces a fuel, also called a "bio-sourced composite," given that charcoal is an energy source.Mixing Binders with Charcoal PowderIt is important to note that the mixing methods for each type of binder with charcoal powder are identical, and the dosages were performed empirically. In our laboratory experiment, we used a bowl for mixing. The previously prepared binders were first poured into the bowl in precise quantities, followed by slow stirring with a spatula. The charcoal powder was added gradually, depending on the homogeneity of the mixture, while increasing the stirring speed. It is crucial that the mixture be neither too dry nor too wet. The proportions of binders and charcoal powder must be balanced to obtain quality briquettes.MoldingMolding consists of pouring the mixture into a mold to obtain the desired briquette shape. This step is essential for adjusting the texture of the mixture and creating a well-defined shape, making the product more aesthetically pleasing. In our case, we used a specific mold for each type of binder.CompactionCompaction is a technique aimed at densifying the briquette through pressure using specialized tools or machinery, making the briquettes stronger. Due to material limitations, compaction was performed manually, using a bottle lid and a small metal plate.Demolding and DryingOnce compacted, the briquette is removed from the mold. Drying is done in the open air, under the sun, by spreading the briquettes on a tarpaulin or, more efficiently, on racks.CO MBUSTION TESTING OF BIO-SOURCED COMPOSITES [20]The objective of this study was to analyze the combustion of the bio-sourced composites produced. This involved not only comparing consumption times, but also visually observing the smoke, hardness, and odors released during the test.Equipment Used:A stove, also called a "fatapera," served as the combustion source. An electronic scale was used to measure the initial mass of each composite. A match was used to light the fire, along with a small piece of pine wood. A stopwatch was used to track the combustion time. The data was recorded on a sheet.Raw materials:100% bio-based composite or briquette.Procedure:Before each test, the fuels were weighed to ensure identical mass. They were then lit and observed until the fire was completely extinguished, recording the start and end times of combustion, as well as the observed phenomena. The "fatapera" had to be empty or clean before each test of these bio-based composites.
4. Results
Polysaccharide extraction methods produce products in powder form. These processes vary depending on the products to be extracted. The yields for each polysaccharide are shown in the following tables:Table 2. Polysaccharide extraction yield
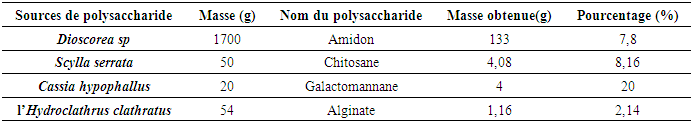 |
| |
|
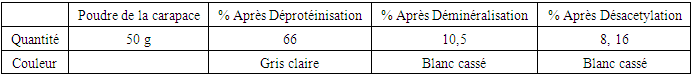
Table 3. Chitosan extraction yield after demineralization and after deacetylation
 |
| |
|
Table 4. Yield of galactomannan after precipitation
 |
| |
|
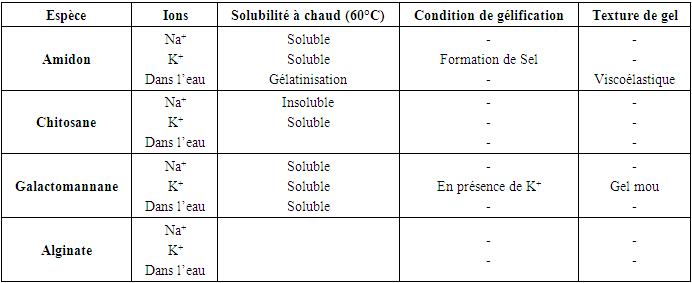
The results obtained are summarized in Table 5.Table 5. Results of gelation test of polysaccharide extracts in the presence of ions (Na+; K+) and in the presence of water
 |
| |
|
 | Figure 14. (A): Bio-based composite based on starch; (B) Bio-based composite based on chitosan, (C): Bio-based composite based on alginate, (D): Bio-based composite based on Cassia gum (Hervéa, 2019 |
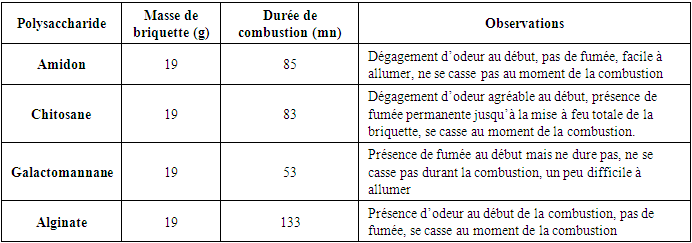
Combustion TestTable 6. Combustion test results
 |
| |
|
5. Discussion
In the starch extraction study, we used a simple artisanal method. The tubers used Were highly toxic, so we first removed the hydrocyanic acid to avoid the risks associated with their use. At this stage, we obtained a starch fraction from Dioscorea sensibarensis with a yield of 7.8%, similar to that observed by Brunnschweiler et al. (2005) during yam starch extraction. According to this study, extraction yields for different yam species range between 8 and 15% relative to fresh material.Regarding chitosan extraction, more complex methods were required. Chitosan was extracted from Scylla serrata shell powder, previously treated with a basic solution for deproteinization. We obtained a polysaccharide fraction with a yield of 8.16% based on 50 g of fresh material. After demineralization and deacetylation, the respective yields were 10.5% and 8.16%.During deproteinization, a decrease in the powder's mass was observed, as all the proteins were removed during the basic treatment. The deproteinized powder was then treated with an acid solution for demineralization, which generated a foam due to the production of carbon dioxide, linked to the presence of calcium carbonate (CaCO3) in the crab by-products. This step explains the absorption of the acid solution by the different layers of the powder. The filtrate was then precipitated with ethanol at room temperature, using three times its volume to obtain chitin, which was then deacetylated using a concentrated base treatment, thus producing chitosan. Galactomannan was extracted by precipitation from the filtrate, with yields reaching up to 20%. To remove lipids, colored constituents, and small molecular impurities, the powder was soaked in a dichloromethane/ethanol solution overnight.For Hydroclathrus clathratus, the extraction was carried out under heat conditions, yielding a polysaccharide fraction with a yield of 1.16% from 54 g of dry leaves. In comparison, the species Ascophyllum nodosum and Laminaria digitata show yields of 15–30% and 20–45% per 100 g of dry matter, respectively (Indegaard, 1991). Thus, our yield is lower than that of other species, demonstrating that the extraction yield depends on the specific conditions used. Gelation TestA study of the physicochemical characteristics was conducted to determine the properties of the polysaccharides, including a gelation test in the presence of different ions (K+, Na+). According to Bouffar (1986), alginate gelation occurs through the formation of physical bonds with calcium ions. Vecino X. et al. (2014) also observed gel formation in the presence of calcium, by stacking the chains according to the egg-box model. Thus, alginate does not gel in the presence of either sodium or potassium. According to Braccini et al. (1999), the strength of the gel formed from alginate depends on the M/G ratio, with a lower ratio resulting in a stronger gel.The gelation test for chitosan showed that it does not gel in the presence of these ions. However, according to Chenite et al. (2001), certain anionic salts such as glycerol, sorbitol, fructose, or glucose-phosphate can induce chitosan gelation. Furthermore, they studied the thermoreversible gelation of chitosan at neutral pH in β-glycerophosphate buffers, where the solution gels upon heating and liquefies upon cooling.Finally, galactomannan gels only in the presence of potassium chloride, forming a soft gel.Physical Appearance of Bio-Based CompositesComparing these four types of composites, we see that the starch-based one is very hard, while the softest is the one made with alginate. The other two composites have an intermediate hardness. Unlike the charcoal we frequently use, these bio-based composites do not leave black marks on the hands. Analysis of extraction rates reveals that the tuber of Dioscorea sensibarensis and the shell of Scylla serrata contain similar amounts of polysaccharides. The seed of Cassia hypophallus contains three times more, while Hydroclathrus clathratus contains four times less. This could suggest that the tuber of Dioscorea sensibarensis contains little starch, which seems surprising given that tubers are generally rich in starch. Indeed, the starch content of starchy plants, Like Dioscorea, the plant's starch content varies with the seasons. During the sap rise, the starch is converted to feed the young shoots, and this period coincides with September, harvest time. To obtain optimal starch levels, it would be best to collect samples just before this phase, during the dormant period. This would allow for the true starch content of the plant to be obtained.For now, Cassia hypophallus appears to be the most interesting material for the production of burning briquettes. As for the Galactomannan-based briquette, although difficult to light, it produces no smoke, which explains its hardness. Furthermore, it does not fragment during combustion, and its burn time is satisfactory. In contrast, the alginate briquette burns slowly but becomes brittle upon combustion. Its interest lies in the amount of energy released during combustion, although this value has not yet been defined. In conclusion, among the materials studied, Cassia hypophallus appears to be the best option as a binder for the production of our bio-based composite.
6. Conclusions
Our study aimed to identify the most reliable composite by combining a polysaccharide with charcoal powder, taking into account their physicochemical properties. We selected four polysaccharide sources: Dioscorea sensibarensis, Scylla serrata shell, Cassia hypophallus, and Hydroclathrus clathratus. First, we extracted the polysaccharides from their respective sources and then prepared their binder by performing gelation tests. All polysaccharides gel, albeit under different conditions. Next, we assembled our bio-based composite, or briquette, and performed a combustion test. It was at this point that we observed that the one bound to galactomannan, based on its observed behavior, was the most resistant. This conclusion is also based on its extraction rate, which is the highest compared to others. The combustion of the starch-bound composite is similar to that of the galactomannan-bound composite, but its extraction yield is low. It would therefore be necessary to harvest Dioscorea sensibarensis during its dormant period to better understand its starch content, although its use remains possible for the preparation of our bio-based composite.The production of our bio-based composite can be industrialized by recovering all organic waste, whether industrial, agricultural, or household, to create a new energy source, with galactomannan as the binder. Thus, humans would adopt a role similar to that of plants, which, through their self-cleaning ecological properties, absorb CO2 from the atmosphere and transform their own waste, thanks to microorganisms, into essential minerals for the production of their substance and their fruits. Similarly, by transforming waste into briquettes, humans create a new source of energy for cooking their food, while eliminating polluting effluents. This productive activity also creates a growing need for galactomannan, thus stimulating the development of Cassia hypophallus cultivation, a new agricultural activity beneficial for rural populations.
References
| [1] | Angellier H. (2005). Nanocristaux d’amidon de maïs cireux pour applications composites. Thèse de Doctorat, Université Joseph Fourier – Grenoble 1 (France). 298p. |
| [2] | Abdulhadi Aljawish. «Fonctionnalisation enzymatique du chitosane par des composés phénoliques: évaluation des propriétés biologiques et physico- chimiques de ces nouveaux biopolymères» Université de LORRAINE (UL). Année 2013. |
| [3] | Andriamiarantsoa Miora. (2017). Les polymères naturels: Fabrication d’un emballage film à base de fécule. Mémoire de fin d’étude en vue de l’obtention du diplôme de Licence en Ingénierie Pétrolière, Université d’Antsiranana, p: 27. |
| [4] | Akita, K., Report of fire Research Institute of Japan, 1959. |
| [5] | Antal, M.J., Croiset, E., Dai, X., Almeida, C. de, Mok, W.L., Norberg, Richard, J.R. et Majtoub, M. High Yield Biomass Charcoal. American Chemical Society, c 1996. |
| [6] | Arbia L., 2010. Contribution à l’étude de la demineralisation et de la déprotéinisation de la carapace de crabe, de la crevette par voie fermentaire, mémoire de magister, ENP, p. 3-12. |
| [7] | Attaie H., ZAKHIA N., BRICAS N. Etat de connaissance et de la recherche sur la transformation et les utilisations alimentaires de l’igname. In: Berthaud J., Bricas N., Marchand J.L. Séminaire international (Montpellier, 3-6 juin 1997). CIRAD; INRA; ORSTOM; CORAF. L’igname, plante séculaire et culture d’avenir, 1998: p 275-284. |
| [8] | Bosh.h, the production and characterization of activated carbon of from coonut shell catalized by potassium carbon. 29: p. 949-953. |
| [9] | Burkill H., PErrier DE LA Bathie. Flore de Madagascar et des Comores (plantes vasculaires). 44è famille de DIOSCOREACEA. Paris: Typographie Firmin Didot et Cie, 1950; 73p. |
| [10] | Bouxin F. 2011. Solvolyse des lignines: production de synthons aromatiques de faibles masses. Thèse de Doctorat de l’Université de Reims Champagne Ardenne. |
| [11] | Buleon A., (2001). Caractéristiques structurales des amidons. Formation Adria des 21 et 22 mars 2001 à Nantes (France). |
| [12] | C. chaussin, Plastiques, DUNOD, Paris, 1961, p: 60. |
| [13] | Carre, J. et al., 1994. Guide Biomasse- Energie, ACADEMIA, Characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydrate Polymers, vol. 46, p. 39-47. |
| [14] | Cheftel JC, Cuq JL, et Lorient D. 1985. Protéines alimentaires. Tec & Doc, Lavoisier, Paris. |
| [15] | Guilbot A., Mercuer C., 1985, Starch, in the polysaccharides, Volume3, Edited by Gerald O. Aspinal, New York, Academic Press. |
| [16] | Glicksman M. (1982). Functional properties of hydrocolloids. In: Glicksman M. (ed): Food Hydrocolloids. CRS Press, Boca Raton, Florida. (V.1), pp. 47-99. |
| [17] | Hirano s., 1996, Chitin biotechnology applications. Biotechnol. Annu. Rev., 2, 237-258. |
| [18] | Hoover r., 2001. Composition, molécular structure, and physicochemical proprieties of tuber and root starches. Carbohydrate polymers, 45: pp. 253-267. |
| [19] | Indegaard, M., Minsaas, J. (1991). Animal and human nutrition in Seaweed Resources in Europe. Uses and Potential, M. D. Guiry and G Blunden (Ed.). Chichester, John Wiley & Sons: 21-64. |
| [20] | J.H. De Boer, J.F.H. Custers, Physica, 1937 (def et propriété du charbon actif charbon vert dossier), 4, 1017. |
















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




