-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2025; 15(1): 1-5
doi:10.5923/j.re.20251501.01
Received: Dec. 19, 2024; Accepted: Jan. 16, 2025; Published: Feb. 6, 2025

Study of Soil Physico-Chemical Parameters for Cocoa Cultivation in the Rural Commune of Marovato in the Ambnaja District of Madagascar
Razafitsiferana Théophile
Université d'Antsiranana, Faculté des Sciences, Mention Chimie, Parcours: Chimie Minérale
Correspondence to: Razafitsiferana Théophile, Université d'Antsiranana, Faculté des Sciences, Mention Chimie, Parcours: Chimie Minérale.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

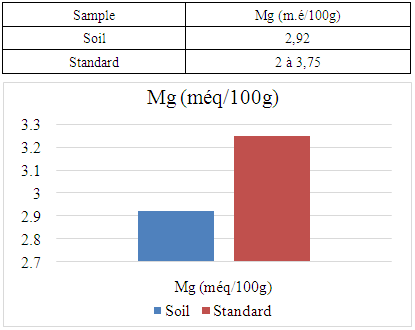
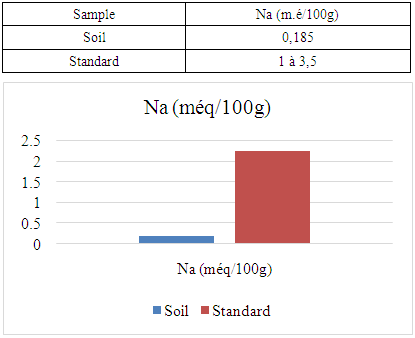
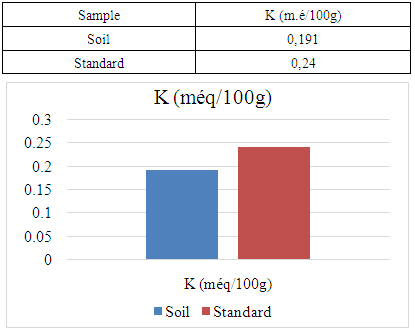
The Ambanja district is the only cocoa-growing district in Madagascar. It is located in the northern region of the island, known as the DIANA region. The aim of my research is to analyze soil physico-chemical parameters in the rural commune of Marovato, in order to identify solutions for improving cocoa crop yields. For the physical parameter, the soil pH is 7.09, indicating a neutral pH. Concerning the chemical parameters: The nitrogen content (N%) is 0.154, indicating that the soil is rich in nitrogen. - The carbon content (C%) is 1.92, indicating that the soil is rich in carbon. - Calcium is 7.00 meq/100g, indicating a very high calcium content. - Magnesium is 2.92 meq/100g, indicating that the soil is rich in magnesium. - Sodium, with a value of 0.185 meq/100g, is low, meaning that the soil is low in sodium. - Finally, the potassium content is 0.191 meq/100g, also indicating that the soil is low in potassium.
Keywords: Soil, Physical parameters, Chemical parameters
Cite this paper: Razafitsiferana Théophile, Study of Soil Physico-Chemical Parameters for Cocoa Cultivation in the Rural Commune of Marovato in the Ambnaja District of Madagascar, Resources and Environment, Vol. 15 No. 1, 2025, pp. 1-5. doi: 10.5923/j.re.20251501.01.
Article Outline
1. Introduction
- The majority of the population of the rural commune of Marovato work on cocoa plantations, which are the main source of income for improving the living conditions of each family. The commune of Marovato is located in the Haut Sambirano region, within the Ambanja district.This is why my research focuses on the physico-chemical parameters of the soil for cocoa cultivation and on ways of improving yields based on the results obtained.
2. Literature Reviews
- Soil type for cocoa: To grow properly, cocoa requires well-structured, permeable, deep soil. Its taproots penetrate deep into the soil, while its secondary roots, located near the surface, bear absorbing hairs responsible for absorbing nutrients. [1], [3], [4]Types of cocoa: The most widely grown variety is Forestero, which accounts for around 79% of harvests. Other varieties include Trinitario and Criollo. [13]Growing period: It takes four to five years after planting for cocoa trees to start producing cocoa beans. [13]Cocoa production cycle: The cocoa tree begins to produce its first fruits, called cocoa pods, around 4 to 5 years after planting. [13]Characteristics of cocoa in the Marovato commune:- First harvest: between 4 and 5 years after planting.- Pruning: cocoa trees generally reach a height of 2 to 3 meters without any particular maintenance.- Harvesting: carried out over a period of 1 to 2 weeks each month.- Fermentation: between 2 and 3 days for standard cocoa and between 5 and 6 days for superior cocoa.- Drying: between 3 and 4 days for standard cocoa and between 5 and 6 days for premium cocoa.Soil parameters: [6], [7], [8]Soil analysis enables us to assess its structure and fertility through four main parameters: texture, acidity, organic profile and mineral status. - Texture analysis: Depending on the different particle classes, several soil types can be identified: sandy, silty, clayey or humus-bearing.- Acidity analysis: The soil's pH determines its level of acidity or alkalinity on a scale ranging from 0 (very acidic) to 14 (very basic or alkaline).- Organic profile analysis: Organic matter (OM) plays an essential role in soil fertility. It is mainly found in the surface layer (0 to 30 cm) and represents around 1 to 10% of total soil mass. [9]- Mineral status analysis: Soil mineral status is defined by the presence of several essential elements, such as nitrogen, carbon, calcium, magnesium, sodium and potassium. [11], [14]
3. Analysis Parameters
- Role of nitrogen:Nitrogen (N) is an essential nutrient for crop growth. As a key component of chlorophyll, it plays a central role in photosynthesis. It is essential to plant structure, plant nutrient metabolism and chlorophyll production.The role of carbon: [9]Increasing carbon content in soils promotes aggregation and structural stability. This directly improves water dynamics and strengthens soil resistance to erosion caused by water and wind. In addition, carbon influences the availability and dynamics of essential nutrients.Role of calcium: [2], [11]Calcium plays an important physical role in stabilizing soil structure. It improves air and water permeability, facilitates root penetration and neutralizes the clay-humus complex, making the soil easier to work.The role of magnesium: [2], [14]Magnesium is often overlooked, even though it is widely used in certain specialized crops. However, it is as essential as calcium to the proper functioning of soils and crops.The role of sodium: [11]Sodium binds to soil particles, causing them to disperse and the clod structure to break down. Like potassium, it plays a key role in plant water regulation, controlling ion concentration in tissues, including stomata. It is also involved in transporting sucrose to the roots.Role of potassium: [11]Potassium strengthens plant cell walls, increases leaf surface area and chlorophyll content, thus delaying leaf senescence. It thus helps improve photosynthesis and stimulate crop growth. [14]
4. Materials and Methods
- For pH: 50 ml beaker, buffer solution ph 4and ph7, weigh 10 g of air-dried soil into a 50 ml beaker, leave in contact for 30 min and pH meter.Ca2+, Mg2+, Na+ and K+ cations:The soil is contacted with a neutral, molar solution of ammonium acetate.Exchangeable base cations are extracted into the solution, while some NH4+ is adsorbed by the soil, according to the equilibrium below:Sol-M + NH4+ (solution) → Sol-NH4+ + M (solution)M: exchangeable base cation.- The extracted base cations are then determined using an atomic absorption spectrometer.- Place 10 g of soil in a 125 ml Erlenmeyer flask. Add 40 ml of 1M ammonium acetate.- Swirl and let stand for 1 hour or more.- Transfer the contents of the Erlenmeyer to a funnel lined with filter paper.- Measure with an atomic absorption spectrometer.To measure organic carbon:Organic carbons are oxidized by an excess of potassium dichromate solution, in an acid medium. Reagents: 1N potassium dichromate, 20 ml concentrated sulfuric acid, 0.5N ferrous sulfate, 0.025N ferrous-ortho-phenantroline complex.Procedure:- Weigh approximately 0.5 g of 0.5 mm diameter sol and record the exact weight. Transfer to a 250 ml Erlenmeyer flask.- Add 10 ml of 1N potassium dichromate and swirl the Erlenmeyer to disperse the soil in the solution.- Quickly add 20 ml of concentrated sulfuric acid.- Leave to stand for 30 mins, add 200ml distilled water, add 4 drops of ortho-phenantroline and titrate the solution with FeSO4 0.5N.- At the end of the reaction, the color changes from intense green to purplish red.Calcul:Carbone organique (%) =
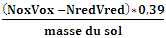 With Ox: potassium dichromateRed: ferrous sulfateNitrogen Principle:- The substance is heated with concentrated sulfuric acid, which, when boiling, destroys the nitrogenous organic matter.- Carbon and hydrogen are released as CO2 and H2O, while nitrogen, transformed into ammonia, is fixed by sulfuric acid as (NH4)2SO4.Reagents:- Concentrated sulfuric acid- Kjeltab mineralization catalyst- 10N sodium hydroxide solution- 0.01N sulfuric acid solution- Mixed indicator- 2% boric acid solutionProcedure:- In a digestion tube, successively introduce 1g of 0.5mm-diameter soil, 1 mineralization catalyst and 10ml of concentrated sulfuric acid.- Heat vigorously (approx. 430°C) for 30 minutes.- After cooling for a few minutes.- Make up to the mark with distilled water.
With Ox: potassium dichromateRed: ferrous sulfateNitrogen Principle:- The substance is heated with concentrated sulfuric acid, which, when boiling, destroys the nitrogenous organic matter.- Carbon and hydrogen are released as CO2 and H2O, while nitrogen, transformed into ammonia, is fixed by sulfuric acid as (NH4)2SO4.Reagents:- Concentrated sulfuric acid- Kjeltab mineralization catalyst- 10N sodium hydroxide solution- 0.01N sulfuric acid solution- Mixed indicator- 2% boric acid solutionProcedure:- In a digestion tube, successively introduce 1g of 0.5mm-diameter soil, 1 mineralization catalyst and 10ml of concentrated sulfuric acid.- Heat vigorously (approx. 430°C) for 30 minutes.- After cooling for a few minutes.- Make up to the mark with distilled water.5. Measurement Results
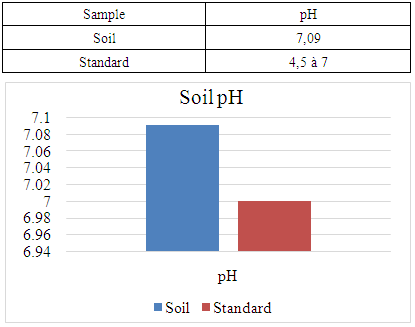
- a- Physical parameterspH:The pH of the soil is given in Table 1 below.
|
|
|
|
|
|
|
6. Interpretation of Results
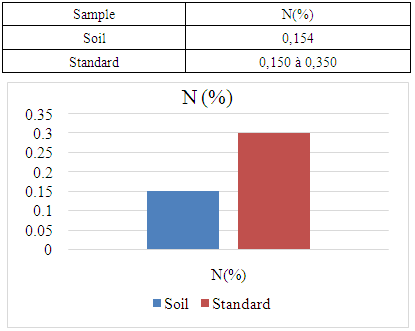
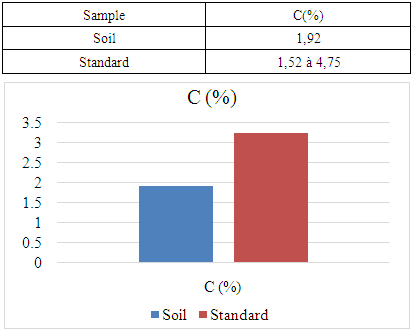
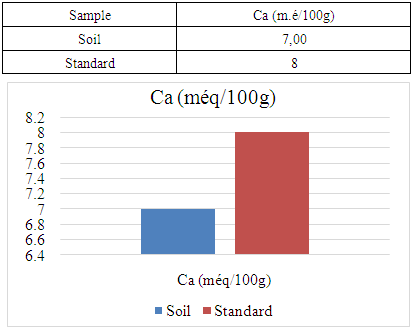
- For the physical parameter: The soil pH measured was 7.09, which is almost neutral. This value complies with the standards required for cocoa cultivation, the acceptable range being between 4.5 and 7.For chemical parameters:- Nitrogen (N%): The value obtained is 0.154%, which complies with the admissible limit of between 0.150% and 0.350%. Thus, the nitrogen content of the soil in the Marovato Rural Commune complies with the requirements for cocoa cultivation.- Carbon (C%): The value recorded is 1.92%, within the permitted range of 1.52% to 4.75%. The soil carbon content of the Marovato Rural Commune is therefore in line with cocoa-growing standards.- Calcium: The measured value is 7.00 m.e/100g, with a maximum limit of 8.00 m.e/100g. The calcium concentration in the soil of the Marovato Rural Commune is therefore acceptable for cocoa growing.- Magnesium: The concentration recorded is 2.92 m.e/100g, within the permitted range of 2 m.e/100g to 3.75 m.e/100g. The magnesium content of the soil in the Marovato Rural Commune therefore complies with the standards required for cocoa cultivation.- Sodium: The concentration found was 0.185 m.e/100g, well below the accepted limit of between 1 m.e/100g and 3.5 m.e/100g. Consequently, sodium levels in the soil of the Marovato Rural Commune are acceptable for cocoa cultivation.- Potassium: The concentration measured is 0.191 m.e/100g, for a limit set at 0.24 m.e/100g. The potassium content of the soil in the Marovato Rural Commune therefore complies with the requirements for cocoa cultivation.
7. Conclusions
- In the light of these results, we can conclude that the four soil analysis parameters - texture, acidity, organic profile and mineral status - meet the required standards for soil quality for cocoa cultivation.However, a number of shortcomings were observed, particularly in terms of sodium and potassium concentrations.At our next meeting, therefore, I'd like to suggest a method for improving these concentrations in order to achieve satisfactory yields.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML