-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2021; 11(1): 1-8
doi:10.5923/j.re.20211101.01
Received: Dec. 14, 2020; Accepted: Jan. 9, 2021; Published: Jan. 25, 2021

Assessment of the Risk of Contaminating Soil Cultivation Fruits and Vegetables by Linuron Residues in the Market Gardening Zone in Marza in Ngaoundere – Cameroon
Thomas Assokeng1, Guy B. Noumi2, Henriette Z. Adjia1, Joseph M. Sieliechi1
1Department of Applied Chemistry, National School of Agro-Industrial Sciences of University of Ngaoundere, Ngaoundere, Cameroon
2Department of Chemistry, Faculty of Sciences, University of Ngaoundere, Ngaoundere, Cameroon
Correspondence to: Guy B. Noumi, Department of Chemistry, Faculty of Sciences, University of Ngaoundere, Ngaoundere, Cameroon.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

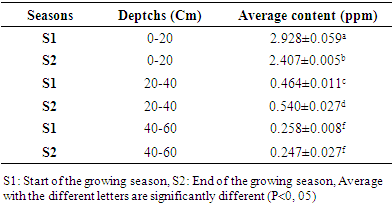
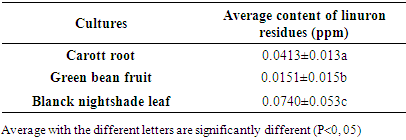
The aim of this work presents the study of the risk of transfer of linuron residues in the soils and vegetables cultivated in the market gardening zone of Marza in Ngaoundéré-Cameroon. The methodology chosen consisted of sampling the cultivated soils at three different depths: 0-20; 20-40 and 40-60cm and three vegetables grown regularly: carrot roots (Daucus carotta), green bean (Phaseolus vulgaris L) and black nightshade (Solanum nigrum) leaves. After validation of the linuronanalysis parameters by HPLC-UV, the linuron residues were extracted from soil samples by magnetic stirrer and purified on a silica gel column. In the meantime, the QuEchERS method has been used for the extraction and purification of linuron residues in samples of fresh fruits and vegetables. The residual concentration of linuron has always been determined by means of HPLC-UV analysis. For the analysis parameters, the correlation coefficient was 0.996 and the detection limit was 0.002ppm. It was found that the linuron residues in 0-20 cm were significantly higher than the other depths, ie. 2.407 to 2.928 ppm for 0-20 cm against 0.464 to 0.540 ppm for 20 to 40 cm and 0.247 to 0.258 for 40 to 60 cm. In vegetables, the average residue levels of linuron from Solanumnigrum, Daucuscarotta roots and Phaseolus vulgaris L were 0.072 ppm, 0.042 ppm and 0.017 ppm, respectively.
Keywords: Linuron, HPLC-UV, Marza soil, Market gardening, Vegetables
Cite this paper: Thomas Assokeng, Guy B. Noumi, Henriette Z. Adjia, Joseph M. Sieliechi, Assessment of the Risk of Contaminating Soil Cultivation Fruits and Vegetables by Linuron Residues in the Market Gardening Zone in Marza in Ngaoundere – Cameroon, Resources and Environment, Vol. 11 No. 1, 2021, pp. 1-8. doi: 10.5923/j.re.20211101.01.
Article Outline
1. Introduction
- The high demand for quality and quantity of fresh fruits and vegetables in the cities of developing countries in general and in Cameroon in particular has led to an intensification of market gardening activities in the lowlands and marshy valleys [1,2,3]. This activity offers a fairly wide range of advantages, namely: a source of income for many housewives and employment for poor populations coming from the countryside. Faced with numerous parasitic attacks, at the high cost of labor; market gardeners are increasingly using many agricultural inputs and in particular phytosanitaryproducts [4,5]. Among these phytosanitary products, herbicides represent around 46.3% of pesticides used worldwide [6]. Among these pesticides, the linuron-based formulations discovered in the 1960s for the control of weeds on tubers (carrots, potatoes, beets) are today in great demand by market gardeners in developing countries in order to drastically reduce the cost of labor. However, many studies have highlighted its high toxicity, its cancerogenic nature [7] and its impact on the decline in male fertility [8,9]. Therefore, the identification and quantification of pesticides in food and environment are increasing public interest. For this reason, very sensitive analytical methods such as high performance liquid chromatography (HPLC) are very important for the identification and quantification of pesticide residues in food and the environment in order to assess the hazards to which populations are exposed [10]. Despite the benefits of this herbicide, the fate of its residues in environmental and food matrices remains today a major problem for the protection of the environment and human health.Indeed, some research has shown contamination of generally developed crops and even resistant crops such as carrots on soils that have undergone a prior treatment with linuron [11]. On the other hand, other studies carried out in Canada have show that the use of formulations based on linuron did not present any risk of contamination for resistant crops such as carrots but rather a risk for sensitive crops such as lettuce and onions [12]. Other studies on the dynamism of linuron in soils have shown an accumulation of residues at the horizon of 0 to 20 cm on clay loam soils. [13]. It seems obvious that extensive investigations seem to be necessary in order to clarify the impact of linuron on local crops and on different types of soils.In Cameroon, Ngaoundere is considered as one of the largest vegetable production areas thanks to its many rivers that do not dry out throughout the dry season and its ability to supply not only local markets with fruits and vegetables but also other towns such as: Garoua and Maroua and towns in neighboring countries such as Ndjamena in Chad and Kartoum in Sudan [5]. Studies carried out on cultivable soils in this town have shown that the use of atrazine led to an accumulation of their residues in vegetables (folon and kelenkelen) [14]. Moreover, other studies have shown an accumulation of maneb residues in regularly grown tomatoes in the market gardening areas of Ngaoundere [15]. However, there is no knowledge on the fate of linuron-based formulations which are in great demand to fight against weeds on carrot crops although several studies have highlighted its carcerogenic nature and its impact on soils, cropsand waters.Among the carrot production basins in Ngaoundéré, the flood-prone area of Marza is considered the main production area because of the fertility of its soils due to the fact that this area was the former pastoral center of theAdamaoua region. This crop is regularly developed by combining with green beans and black nightshade. However, the increasing use of linuron-based formulations raises a lot of concerns about the health quality of regularly grown plants and about the dynamism of cultivated soils. Assessing the risks of contamination of crops and soils in market gardening areas of Marza by residues of this pesticide remains a major concern today, and the dangers to which consumers and the environment are exposed must be addressed. Thus, the aim of this study was to assess the risks of transfer of linuron residues in cultivated soils and crops (carrots, green beans and black nightshade) regularly developed in the Marza vegetable production basin in a specific context to market gardeners.
2. Materiel and Methods
2.1. Technical Itinerary of the Plot Studied
- The technical itinerary of the plot studied was carried out by the market gardeners themselves. For, 8 furrows were formed for the development of crops. After the carrot seeds were spread, the plot was irrigated every morning using a watering can. 4 weeks after the emergence of the carrot seedlings, a 0.1mg/L solution of commercial linuron (tromisil 350mg/kg) was prepared by the market gardeners and applied using a sprayer. Two days after application, the green bean kernels were sown at the edge of the plots and the nightshade seedlings were transplanted. Thereafter, watering was carried out every morning and the plants were followed in their growth until maturity where they will be sampled.
2.2. Chemical
- Linuron (99% purity) was obtained from Sigma Aldrich, Germany. Methanol, acetonitrile were suppied by Carlo Erbo commercial and linuron(Tromisil 350g/Kg) was purchased from sellets of pesticides in Ngaoundere (Cameroon). The distiller water used was filter with a membrane filter of 0.2 μm. The QuErchERSSupel ™ QuEPSA / ENVI-carb ™ SPE kits used for the cleanup of linuron residues in vegetables were obtained from Sigma Aldrich, German.
2.3. Chromatographic Analysis
- The analysis was carried out by the HPLC-UV brand Shimadzu (LC20A), equipped with four organic solvent pumps and coupled to a UV adsorption detector with an array of photodiodes (SPD-M20A, 190-800 nm). A C18 in reverse phase (int ø: 4.6 mm, length 150 mm) was used as analytical column, and the temperature of the column was maintained at 25°C. The whole was controlled by an hp computer equipped with the software. Operation and data acquisition"Lab Solution". Acetonitrile in distilled water (60:40) was used as the mobile phase with a flow rate of 0.8 ml / min. Prior to HPLC analysis; samples were filtered through 0.2 μm nylon syringe filters. Chromatograms were obtained after manual injection (20 μL) of standard solution and samples. UV detection was performed at a wavelength set at 254 nm. For the preparation of the calibration curve, equal volumes of several different concentrations varying from 0.02 to 0.32 ppm of standard 99% purity linuron solution supplied by Sigma-Aldrich were injected into the HPLC machine. The detection limit was obtained by diluting the lower concentration of the calibration curve (0.02 ppm) until no further signal was detected. For this, the concentrations varying from 0.001; 0.002; 0.005; 0.01 and 0.02 ppm were injected. For the specificity of linuron, 0, 1 mg / L solution of tromisil was prepared to confirm the presence of linuron in commercial formulations regularly used by farmers.
2.4. Sampling
2.4.1. Sampling Zone
 | Photo 1. Sample of cultivable soil of Marza |
2.4.2. Collection and Preparation of Samples
 | Photo 2. Carrots root |
 | Photo 3. Green bean fruit |
 | Photo 4. Black nightshade leaves |
2.4.3. Physical Characteristics of Soil Samples
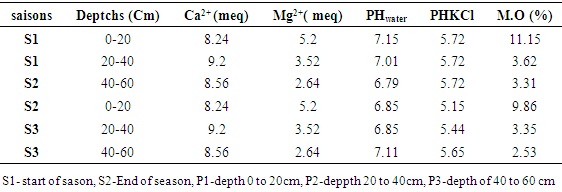
- The PHH2O of the samples were measured using a pH-meter fitted with a glass electrode 24 hours after mixing 2 g of soil from each soil sample in 20 mL of distilled water. At the same time, the pHKClwere measured after having introduced 2 g of soil into 20 mL of 1N KCl solution [17,18]. The organic matter content of the soil samples was determined by the loss on ignition method [19]. A mass of 5 g of each soil sample dried at 105°C in an oven for 24 hours was incinerated at 375°C in a muffle furnace for 16 hours, then the organic matter (OM) content was deduced by gravimetry according to the equation below.
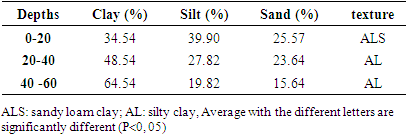
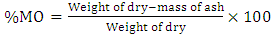 The exchangeable bases Ca2+ and Mg2+ were extracted from the soil with a solution of ammonium acetate. For this, 20g of soil were poured into a 100mL beaker. After addition of 40 mL of a 1M ammonium acetate solution at pH = 7, the mixture was stirred for 5 minutes and left to stand for 5 hours before filtration. Subsequently, 10 mL of extract were assayed for Ca2+ ions and Mg2+ were determined by complexometry using a 1N EDTA solution [20].The granumelotry of the soil was determined by the hydrotimetry method. After determining the granumelotry, the soil texture was deduced from the texture triangle [21,22].
The exchangeable bases Ca2+ and Mg2+ were extracted from the soil with a solution of ammonium acetate. For this, 20g of soil were poured into a 100mL beaker. After addition of 40 mL of a 1M ammonium acetate solution at pH = 7, the mixture was stirred for 5 minutes and left to stand for 5 hours before filtration. Subsequently, 10 mL of extract were assayed for Ca2+ ions and Mg2+ were determined by complexometry using a 1N EDTA solution [20].The granumelotry of the soil was determined by the hydrotimetry method. After determining the granumelotry, the soil texture was deduced from the texture triangle [21,22].2.5. Extraction of Linuron
2.5.1. Extraction of Linuron Residues in Soil Samples
- A test portion of 1g of suitable ground soil, sieved at 2mm, was introduced into a 100mL Erlenmeyer. After adding 10 mL of methanol, the mixture was brought to the magnetic stirrer for 2 hours. Subsequently, the mixture was centrifuged at 3500rpm for 5min. After centrifugation, the supernatant has been purified through a column. The purification was carried out on silica gel in column. Concretely, it was about introducing 5g of silica gel into the column with a weight of 3g of of anhydrous sodium sulphate. This pre-elution was followed by elution of the suspension (linuron extract) which was carried out using a mixtures of solvent consisting of acetone and methanol (50:50). The eluent was collected and filtered through a 0.2μm membrane filter then injected with HPLC-UV from Shimazu (LC20A).
2.5.2. Extraction of Linuron Residues in Fruit and Vegetable Samples
- The QuEchERS (Quick, Easy, Cheap, Effective, Rugged and Safe) method used for the extraction and purification of linuron residues in food was developed in 2003 [23]. Each sample (10g) of fresh vegetable was cut marcerated and homogenized in motar into a 50mL Teflon centrifuge tube, then 10 mL of acetonitrile was added. The overall solution was shaked vigorously for 1min, and 4g of magnesium sulfate anhydrous (MgSO4), 1 g of sodium chloride (NaCl). The tube was closed, shaken vigorously for 1min and centrifuged for 5min at 3000 rpm. Transfer a 6mL aliquot of the upper layer into a 15mL Teflon centrifuge tube containing 150mg PSA, 900mg MgSO4 and 45mg of carbon black (Envi-Carb ®). The tube was closed, shaken vigorously by vortex for 1min and centrifuged for 5min at 3000rpm. Each final extract was then filtred through a 0.2μm membrane filter into a 1.5mL amber glass vial for HPLC-UV analysis.
2.6. Data Analysis
- Stat graphics 2007 software and Excel 2007 were used to perform the ANOVA text on the average residue levels of linuron in the samples. The XLSTAT 2007 software was used to carry out the PCA between the physico-chemical parameters of the soils and the content of linuron residues.
3. Results and Discussion
3.1. HPLC-UV Analysis
3.1.1. Linearity and Limit of Detection
- A calibration line was obtained after analysis of the standard solutions with equation y = 65861x + 8689 and R2 = 0.996. Coefficients of determination (R2) were higher than 0.99, which indicate that regression line perfectly fits the experimental data, showing a linear response over concentration. Regarding the detection limit, after injection of 0.02; 0.005; 0.002; 0.001 ppm. The detection limit was 0.002ppm. This value is of the same order of magnitude as that found in 2016 during a study on validation of the method for analyzing diuron residues by HPLC-UV. In addition, this limitation is similar to that found in a study investigating the determination of linuron in charman by LC-MS [24].
3.1.2. Speciation of Linuronby HPLC
- The chromatograms of standard linuron and a commercial formulation of linuronare presented respectively in the figures 1 and 2. Figure 2 confirms that the commercial formulation of the name tromisilsold in shops in Ngaoundere and used in market gardening areas contains linuron. The retention time, which is 6.08 minutes, is less than the retention time of 8.02 minutes obtained during a study on the photocatalytic degradation of linuron by inorganic materials doped with artificial light and the sun, which is 8,5mn [25]. This observation would be due to the fact that we used the flow rate of 0.8mL/min while it had used the flow rate of 1mL / min.
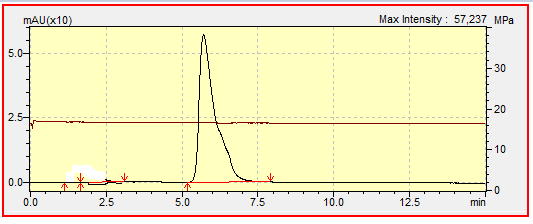 | Figure 1. Chromatogram of standard linuron (0.2ppm) |
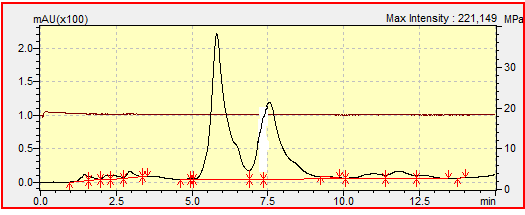 | Figure 2. Chromatogram of a commercial formulation of linuron (Tromisil 350g/Kg) at 0,1mg/L |
3.2. Content of Residues of Linuron in Soil Samples and Physical Characteristics of Soil Sample
- Table 1 presents the levels of linuron residues in soils at different depths depending on the season. It emerges from this table that at the start of the growing season, the average content of linuron residues is greater than the content at the end of the season for depths varying from 0 to 20 cm. The drop in the content at the end of the season can be justified by the fact that this season is marked by the return of the rains and consequently the residues of the runoff water have carried some of the linuron residues towards surface water. These results corroborate with studies carried out by the Environmental Protection Agency which have shown that linuron residues are practically immobile in soils and that in the rainy season; the runoff water carries them more towards surface water [26]. At the depth varying from 20 to 40 cm, it is observed that the content of linuron residues at the start of the season is lower than the content of residues at the end of the season. This may be justified by the fact that linuron residues have migrated from the horizon varying from 0 to 20 cm to depths of 20 to 40 cm. At the depth varying from 40 to 60cm, there is no difference between the content of linuron residues and the end of the season. By comparing the contents according to the depths, it was observed that the content of linuron residues at the level of the depth varying from 0 to 20 cm is greater than the other depths (20 to 40 and 40 to 60 cm). The high content at the level 0 to 20 cm depth can be justified by the fact that the organic matter content at the 0 to 20 cm horizon is greater than at other depths (Table 2). linuron and the physicochemical parameters of soils, we observe a strong correlation of 0.99 between organic matter and the content of linuron residues (figure 1). These results are similar to studies conducted on linurontransfer in cultivated soils in Canada [27]. These results are also corroborated with studies carried out on the adsorption of linuron on clay modifying with organic matter [28]. The high content of linuron in the depth of 0 to 20cm could also be justified by the fact that linuron has a low leaching potential (GUS index is 2.31) and therefore the content will be higher in the depth of 0 at 20cm [29]. Moreover, The Koc value for soil is 670 (log Koc=2.83), which indicates a moderate adsorption potential and a moderate mobility [30]. By observing the texture of the different soil depths, we observe that the depth varying from 0 to 20 cm is of type ALS and the other depths are AL. In these different depths, the linuron residues were detected. These results are different from the studies, which showed beyond the cultivable horizon, linuron residues are no longer detectable [31]. This contradiction would be due to the fact that the cultivable horizon is ALS while the other was A.
|
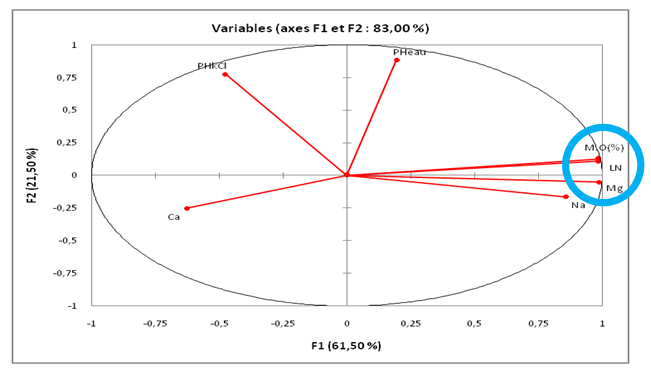 | Figure 3. Correlation between the Physical characteristics of soil and the content of linuron residues |
|
|
|
4. Conclusions
- The objective of this study was to evaluate the risk of transfer of residues of linuron in cultivated soils, fruits and vegetables fresh in the area market gardening Marza. It from this work that the use of linuron- based formulations (tromisil 350g/Kg) in the Marza market garden production basin leads to the contamination of cultivated soils and of the three regularly developed plants. The high content of linuron residues in the depth varying from 0 to 20 cm willconstitute a risk for regularly cultivated plants and micro-organisms responsible for soil fertility. The presence of residues of linuron in art leaves the black nightshade, carrot and green bean roots fruit regularly developed to value than the FAO/ WHO norm of 0.001ppm deriving their a serious risk of contamination for the consumer and could be at the origin of many carcinogenic diseases.
Conflict of Interests
- The authors declare that there is no conflict of interests regarding the publication of this paper.
ACKNOWLEDGEMENTS
- The authors wish to thank the market gardeners of Marza that accepted to collaboration and gave the site to this study, Laboratory of active substance and Pollution to The National School Agro-Industry and Laboratory to Chemical Inorganic of Faculty of Science of University of Ngaoundere for helping with analytical determination.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML