S. D. Thennakoon 1, K. A. Renuka 2, M. G. T. S. Amarasekara 1, J. A. M. H. Jayawardhane 1
1Faculty of Agriculture, Rajarata University of Sri Lanka, Puliyankulama, Anuradhapura, Sri Lanka
2Field Crop Research and Development Institute, Mahaillupallama, Sri Lanka
Correspondence to: M. G. T. S. Amarasekara , Faculty of Agriculture, Rajarata University of Sri Lanka, Puliyankulama, Anuradhapura, Sri Lanka.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Chilli (Capsicum annum L.) is an important spice crop grown in Sri Lanka. Deficiency of micronutrients, especially Mn, Zn and Cu reported in chilli growing areas has affected adversely on chilli production. Therefore, a study was conducted to evaluate the effect of foliar application of Mn, Zn and Cu on growth and yield of chilli grown in Reddish Brown Earth soils (Rhodustalfs), in dry zone, Sri Lanka. Seven treatments were tested in a greenhouse as pot experiment. Nitrogen, phosphorus and potassium were added into all seven treatments. Manganese Zn and Cu were added separately as foliar application for three treatments. One treatment had all three elements and control treatment had only N, P and K but no any added elements. Since all micronutrients were added as their sulfate form, last two treatments had S with N, P and K to assess the effect of S on plant growth. Copper with N, P, K added treatment showed significantly higher growth and yield compared to other treatments. It showed about 25% yield increment compared to control treatment. Therefore, it can be concluded that application of Cu is beneficial to obtain a better yield in chilli in Reddish Brown Earth Soils (Rhodustalfs). However, further field studies are needed to confirm findings.
Keywords:
Chilli, Copper, Foliar application, Manganese, Zinc
Cite this paper: S. D. Thennakoon , K. A. Renuka , M. G. T. S. Amarasekara , J. A. M. H. Jayawardhane , Effect of Foliar Application of Manganese, Zinc and Copper on Growth and Yield of Chilli (Capsicum annum L.), Resources and Environment, Vol. 10 No. 3, 2020, pp. 41-45. doi: 10.5923/j.re.20201003.01.
1. Introduction
Chilli is an important cash crop grown in Sri Lanka. It is extensively grown for dry chilli production, but part of the crop is harvested as green pods. Chilli can be grown throughout the year in all agro-climatic regions in the country. Department of Agriculture of Sri Lanka has recommended several chilli varieties namely MI -1, MI -2, KA -2, MI -Hot, Galkiriyagama selection and Arunalu for green and dry chilli production. Per capita consumption of chilli in the form of dry chilli has been estimated as 2.8 kg per annum and the national annual requirement of dry chilli is around 57,500 tons (FCRDI, 2018). However, the annual production of dry chilli in Sri Lanka is about 7,500 tons. Therefore, approximately 50,000 tons of dry chilli are imported annually. The potential yield of these varieties is 2.5-3.0 t/ha, However, the national average yield is about 1.0-1.5 t/ha (FCRDI, 2018). This yield reduction is mainly attributed to the pest and disease and soil fertility related issues, especially inadequate supply of nutrients. Plants need both macro and micro nutrients to complete the life cycle. Macronutrients are required in larger quantities compared to micronutrients which are required in lesser quantities (Benepal, 1967). Macronutrients are often provided as fertilizers. But only source of micronutrient available in the soil is the organic matter which is incorporated into the soil as organic manure. There are seven nutrient elements defined as micronutrients (Brady and Weil, 2002). Among them Mn Zn and Cu are essential for many plant metabolic processes varying from cell wall development to respiration, photosynthesis, chlorophyll formation, enzyme activity and nitrogen fixation (Ballabh et al., 2013). Therefore, proper plant nutrition through micronutrient is one of the important factors in improving the growth, yield of chilli. Deficiency of micronutrients can be occurred due to multiple reasons such as intensive cropping, with introduction of high yielding varieties, loss of micronutrients by leaching and decreased use of organic manure. It is realized that productivity of crop is being adversely affected due to deficiencies of micronutrients (Bose and Triathi., 1996). In general, farmers add chemical fertilizers to provide nitrogen, phosphorous and potassium for chilli but hardly ever apply micronutrients such as Mn, Zn and Cu. This has resulted reduction of micronutrient availability in the soil. Hence, growth and the yield of the crop can be affected adversely. However, only few studies have been conducted to study the effect of application of micronutrients on chilli. Therefore, this study was mainly focused to evaluate the effect of Mn, Zn and Cu as foliar application on chilli under irrigated condition in the dry zone, Sri Lanka.
2. Materials and Methods
2.1. Location
The study was conducted as a pot experiment at Field Crop Research and Development Institute, Mahaillupallama, Sri Lanka, which is in the Low Country Dry Zone, DL1 agro-ecological region. The altitude of the location is 117m above mean sea level and the mean annual rainfall is about 1750 mm (FCRDI, 2018). Well drained Reddish Brown Earth soils (Rhodustalfs) collected from a bare land were used for the pot experiment.
2.2. Experimental Design and Treatments
The experiment design was Complete Randomized Design with seven treatments and eight replicates. Fifty-six pots were arranged for testing of following treatments.T1- No micronutrients + DOA recommendation NPK (Control)T2 – Mn (MnSO4) + DOA recommendation NPK*T3 – Zn (ZnSO4) + DOA recommendation NPKT4 – Cu (CuSO4) + DOA recommendation NPKT5 – Mn (MnSO4) + Zn (ZnSO4) + Cu (CuSO4) + DOA recommendation NPKT6 – S powder (Avg S amount of MnSO4, ZnSO4 and CuSO4) + DOA recommendation NPK (for testing the effect of added S in T2, T3 and T4 treatments)T7 – S powder (Total S amount of MnSO4, ZnSO4 and CuSO4) + DOA recommendation NPK (for testing the effect of added S in T6 treatment)* DOA recommendation NPK- Department of Agriculture of Sri Lanka recommended rate of N, P and K for chilli
2.3. Nursery Management
A land with well-drained soil was selected for nursery preparation. It was ploughed manually and soil was incorporated with well decomposed farm yard manure. The nursery bed was sterilized by burning of paddy husk and straw. Chilli seeds (variety KA – 2) were established after treated with a fungicide. Paddy straw was spread over the nursery bed to prevent excessive moisture loss. Nursery was wetted daily at twice. Seven days after the seed establishment, mulch was removed and made cadjan shelter over the nursery. Seedlings were steadily exposed to the direct sunlight by removing shelter gradually.
2.4. Crop Establishment and Management
After four weeks, seedling was transplanted in plastic pots (24cm×30cm) which have filled with well drained, RBE soils. Basal dressing was added to the pots two days before transplanting. Equal watering and fertilization according to the Department of Agriculture (DOA) recommendation were followed (Table 1).Table 1. Fertilizer application rates
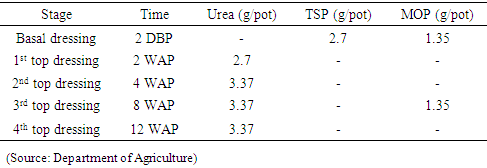 |
| |
|
The micronutrient application was done according to the rate given in table 2. All three micronutrients were applied as foliar applications in 2, 4, 6 and 8 weeks after transplanting at the rate of 100 ml at one application. Table 2. Micronutrient application
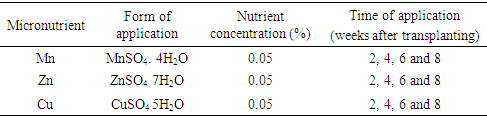 |
| |
|
Manual weeding was done before fertilizer application. Other cultural practices such as loosening up of soil and earthing up of soil were carried out at 4th and 8th weeks after planting. Chilli leaf complex was found in every growth stages of cultivation. Recommended chemicals were applied in order to mitigate casual agents for chilli leaf curl, such as thrips, mites, aphids and white flies. Chilli pods were harvested at green mature stage.
2.5. Sampling and Sample Analyses
In order to determine initial soil characteristics, four soil samples were obtained (0-15 cm depth) from the field area where soil collected for filling pots. Soil samples were obtained from each treatment at after harvesting. Collected samples were air dried on polythene sheet at room temperature. Visible debris like stone, plant parts were removed, and sample was crushed to break the aggregated structure. Then soil was passed through 2mm sieve and sieved samples were collected into labeled polythene bags. Samples were oven dried at 60°C and were grinded prior to analyses. Prepared soil samples were analyzed for chemical parameters such as pH, Electrical Conductivity by Potentiometry using Multi parameter analyzer. Available P by Ascorbic acid colorimetric method (Olsen et al., 1954), using UV Spectrophotometer. Exchangeable potassium by NH4OAc extraction method using flame photometer. Available Sulphur was analyzed by turbidimetric method (Chapmen et al., 1961). Total Nitrogen by Kjeldhal titrimetric procedure (Bremner et al., 1982). Organic matter content by titrimetric method (Walkley and Blank, 1934). Manganese, Zinc, Copper of plant and soil was determined by DTPA extraction method (Lindsay and Norvell, 1978).Leaf samples from the plants in each treatment were obtained at first harvesting for determination of Mn, Zn and Cu uptake.
2.5.1. Growth Parameters
Selected agronomic parameters such as plant height at 50% flowering stage and 1st harvesting stage, canopy width at 50% flowering stage and 1st harvesting stage, number of pods per plant and green chilli yield were measured.
2.5.2. Data Analysis
Collected date were analyzed by the ANOVA using SAS software package. Duncan’s New Multiple Range Test was used for the mean separation of the treatment.
3. Results and Discussion
3.1. Soil Properties and Characteristics at Initial Stages
According to the initial soil analyses, soil pH was 6.9 and EC value was 0.03 dS/m. These values indicated that soil reaction was near neutral and no potential to develop soil salinity. Chilli can grow successfully with soil pH of 5.5 – 7.0 (Mc Collum, 1980). Therefore, soil pH was in line with the crop preferable range. Available N and P were 26 and 15 ppm respectively. However, available K content of 364 ppm showed that it was sufficient for optimum growth of the crop. A moderate level of organic matter content (3.1%) was reported at the initial soil analyses. The availability of Mn, Zn and Cu were 13.4, 21.1 and 2.2 ppm respectively (Table 3). Table 3. Soil characteristics at initial stage
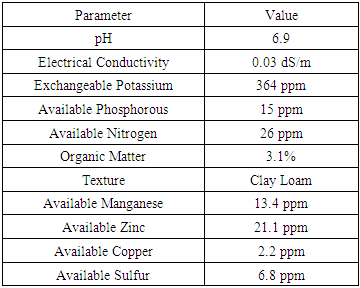 |
| |
|
3.2. Canopy Width at 50% Flowering and First Harvesting
There was a significant difference of canopy width among tested treatments. It varied between 15.7 to 26.3 cm at 50% flowering stage (Table 4). Highest canopy width of 26.3 cm was reported in T4 treatment which had Cu with N, P, K fertilizers. The increment of the canopy width of the T4 was 30.1% compared to canopy with of control treatment (T1). This may probably due to positive response of chilli to added Cu with N, P, K fertilizers. The lowest canopy width of 15.7 cm was observed in T5 treatment. Chilli plants in T5 treatment showed blazing appearance and ultimately fallen of leaves. This may due to the high salt concentration because of the application of all three micronutrient solutions together. As a result, T5 recorded lowest plant canopy width. However, appliance of micronutrients showed significant effect on canopy width at 1st harvesting stage. There was no significant disparity among T1, T2, T3 and T6. Highest canopy width observed in T4 and lowest canopy width in T5 admiration to others (Table 5). Table 4. Canopy width at 50% flowering stage
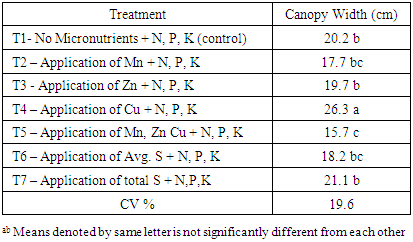 |
| |
|
Table 5. Canopy with at first harvesting
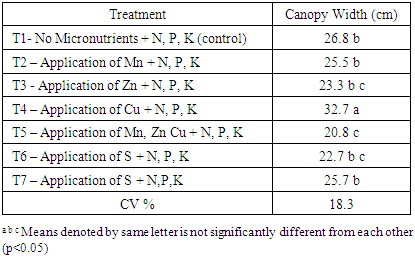 |
| |
|
3.3. Number of Pods per Plant and Fresh Weight
The number of pods per plant is an important yield parameter of chilli to achieve optimum yield. Chilli plants underwent significant variation in number of pods per plant under different treatment conditions. Highest number of pods per plant was observed in T4 while the lowest number of pods per plant was given in T5 treatment (Table 6). Highest canopy width at 50% flowering and first harvesting stage were also reported in T4 treatment. It may increase the bearing points of plant and may help to increase the number of pods per plant in T4 compared to other treatments. Among three micronutrients, Cu added treatment showed highest number of pods per plant compared with others indicating positive response of chilli plants to added Cu. (Patil et al., 2010).Table 6. Number of pods per plant
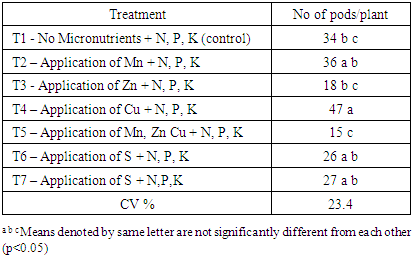 |
| |
|
3.4. Average Fresh Yield
The treatments were significant (p< 0.05) on the fresh yield under different nutrient combinations. A significant increment (109 g/plant) of fresh chilli yield was observed in T4 treatment (Table 7). It showed nearly 25% yield increment compared to control treatment (T1). Increased yield due to Cu application may be attributed to enhanced photosynthesis activity and favorable effect on vegetative growth and retention of flowers and fruits, which increased number of fruits per plant (Dongre et al., 2000). The lowest green chilli yield of 34 g/plant was recorded in T5 treatment. The reason could be retarded growth of T5 treatment due to high salt content adding of three nutrient solutions. Table 7. Average yield
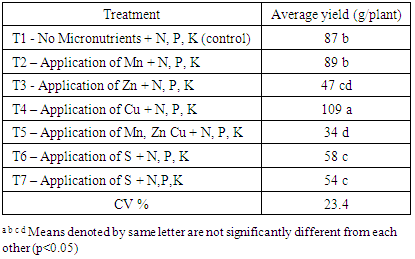 |
| |
|
3.5. Mn, Zn and Cu Uptake at 1st Harvesting Stage
Manganese is a micronutrient essential to plant growth. In plants, it involves in the structure of photosynthetic proteins and enzymes. Excessive accumulation of Mn in plant tissues can change various processes, such as enzyme activity, translocation and utilization of other mineral elements (Ducic and Polle, 2007; Lei et al, 2007). According to literature, the comfortable range of Mn for better plant growth varies between 20 – 200 ppm. Results revealed that no significant difference of Mn uptake at 1st harvesting stage among tested treatments (Figure 1). All the treatments including control were within the sufficient range indicating soil Mn level is sufficient for plant growth. However, treatment T2 and T5 showed increased Mn level (> 30 ppm) compared with other treatments. These two treatments received Mn as foliar application at 2, 4, 6 and 8 weeks after transplanting. Even though zinc uptake was not significantly different, T3 and T5 treatments reported zinc uptake (> 35 ppm) at 1st harvesting stage (Figure 2). Similar to Mn, Zn accumulation in plant leaves could also be due to the foliar application in different stages. Tariq (2007) also highlighted that foliar spray of Zn, Mn significantly increase their concentrations in plant leaves. 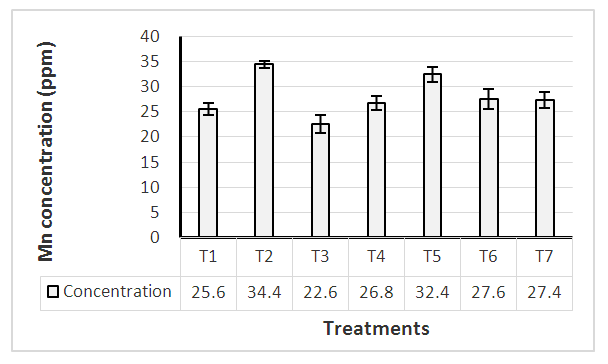 | Figure 1. Mn level of plant leaves at 1st harvesting stage |
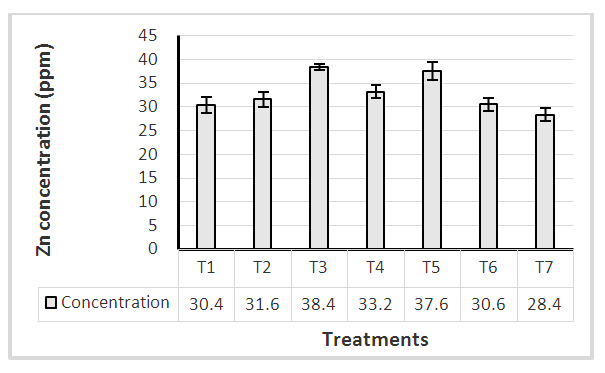 | Figure 2. Zn level of plant leaves at 1st harvesting stage |
Treatment T4 (application of Cu) and T5 (application of Mn, Zn and Cu) were reported higher Cu content (above 10 ppm) in leaves at 1st harvesting stage compared with other treatments (Figure 3). In general, optimum Cu content required for better plant growth is about 10 ppm (Yruela, 2005). Figure 3 shows that other than T4 and T5, Cu content of all the other treatments were below the critical level. Copper as a micronutrient involves in the process of photosynthesis and assists in plant metabolism of carbohydrates and proteins (Droppa and Horváth 1990). As such treatments in which Cu content was below the critical level showed poor performance and lower yields. 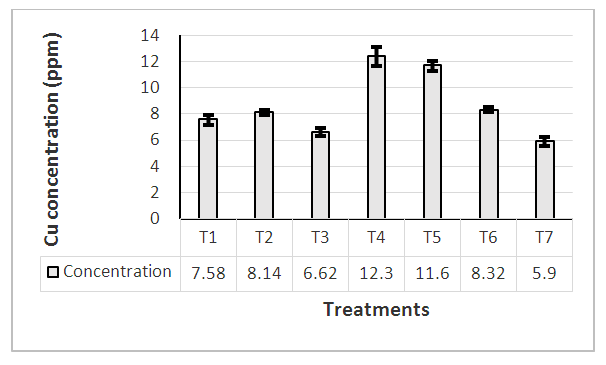 | Figure 3. Cu level of plant leaves at 1st harvesting stage |
4. Conclusions
Foliar application of Cu showed significant increment of green chilli yield but application of other two nutrient did not exhibit any yield improvement. This positive response to added Cu could be due to insufficient amount of Cu available in the soil. However, treatment which had all three elements showed poor performance due to high salt content added as foliar application. Therefore, it can be concluded that application of Cu is beneficial to obtain a better yield in chilli in Reddish Brown Earth Soils (Rhodustalfs). However, further field studies are needed to confirm findings.
References
| [1] | FCRDI (2018). Department of Agriculture. Field Crop Research and Development Institute http://www.gov.lk/ FCRDI. Accessed on 12.08.2018. |
| [2] | Ballabh, K., Rana, D. K. and Rawat, S. S. (2013). Effects of foliar application of micronutrients on growth, yield and quality of onion. Indian J. Horticulture. 70(2): 260-265. |
| [3] | Benepal, P. S. (1967). Influence of micronutrients on growth and yield of potatoes. American Potato Journal, 44(10), 363-369. |
| [4] | Bose, U. S., and Tripathi, S. K. (1996). Effect of micronutrients on growth, yield and quality of tomato cv. Pusa Ruby in MP. CROP RESEARCH-HISAR-, 12, 61-64. |
| [5] | Brady, N.C., Weil, R. (2002). The nature and properties of soils. 13th Edition, Pearson Education, New Jersey. USA. |
| [6] | Bremner, J.M and Muloaney, C.S. (1982). In Methods of soil analysis. Part 2.(2) Agronomy No.09, American Society of Agronomy, Madison, WI, USA. |
| [7] | Chapman, H.D. and Partt, P. F. (1961). Methods of analysis for soil plant and waters. University of Califonia., Div of agric. Sci. |
| [8] | Dongre, S. M., Mahorkar, V. K., Joshi, P. S. and Deo, D. D. (2000). Effects of micronutrients spray on yield and quality of chilli (Capsicumannum L.) var. Jayanti. Agric. Sci. Digest. 20(2): 106-107. |
| [9] | Droppa, M and Horváth, G. (1990). The role of copper in photosynthesis. Plant Sci. 9: 111-123. |
| [10] | Ducic, T. and Polle, A. (2007). Manganese toxicity in two varieties of Douglas fir (Pseudotsuga menziesii var. viridis and glauca) seedlings as affected by phosphorus supply. Funct. Plant Biol. 34, 31-40. |
| [11] | Lei, Y., Korpelainen, H. and Li, C. (2007). Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 68, 686-694. |
| [12] | Lindsay, W. L. and Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Amer. J. 42: 421-428. |
| [13] | McCollum, J. P. (1980). Producing vegetable crops. The interstate printers and publishers, USA, 391-402. |
| [14] | Olsen, S.R., Cole, C.V., Watanabe, F.S and Dean, L.A. (1954). Estimation of available phosphorous in soil by extraction with sodium bicarbonate. USDA Cri. No.939. |
| [15] | Patil, B. C., Hosamani, R. M., Ajjappalavara, P. S., Naik, B. H., Smitha, R. P., & Ukkund, K. C. (2010). Effect of foliar application of micronutrients on growth and yield components of tomato (Lycopersicon esculentum Mill.). Karnataka Journal of Agricultural Sciences, 21(3). |
| [16] | Tariq, M., Sharif, M., Shah, Z., & Khan, R. (2007). Effect of foliar application of micronutrients on the yield and quality of sweet orange (Citrus sinensis L.). Pak. J. Biol. Sci, 10(11), 1823-1828. |
| [17] | Walkley, A.; Black, I.A. (1934). An examination of Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Science 37: 29-38. |
| [18] | Yruela, I. (2005). Copper in plant. Brazilian Journal of Plant Physiology, 17(1), 145-156. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





