Razafitsiferana Théophile, Razanamparany Bruno
University of Antsiranana, Faculty of Sciences, Chemical Sciences, Mineral Chemistry, Madagascar
Correspondence to: Razafitsiferana Théophile, University of Antsiranana, Faculty of Sciences, Chemical Sciences, Mineral Chemistry, Madagascar.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
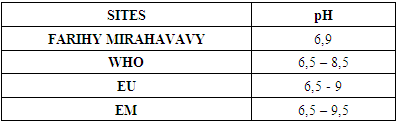
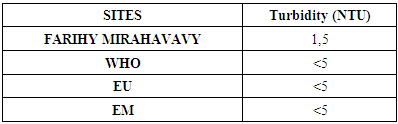
The physical parameters: the conductivity is 250 µS / cm, the turbidity is 1.5 NTU, the pH is 6.9 and the temperature is 20.5°C. The four physical parameters exactly meet the standards required for drinking water. Physical parameters: mineralization is 158 mg / L, total hardness is 20.25 ° f, calcium is 45 mg / L, magnesium is 15 mg / L, potassium is 6 mg / L , sodium is 85 mg / L, chloride is 130 mg / L, total iron is 0.15 mg / L, lead is 0.04 mg / L, and aluminum is 0.02 mg / L. The concentration of the chemical parameters found are admissible to international standards for water intended for human consumption, despite the insufficiency of some concentrations such as calcium, potassium and magnesium.Microbiological parameters: microorganisms at 22°C are 200 MPN / mL, microorganisms at 36°C are 35 MPN / mL, coliform bacteria 15 MPN / 100mL, Escherichia coli is 5 MPN / 100mL and enterococci gut is 2.5 MPN / 100mL The microbiological parameters found are very bad for drinking water.
Keywords:
Physico-chemical, Microbiological and disinfection parameters
Cite this paper: Razafitsiferana Théophile, Razanamparany Bruno, Study of Physicochemical and Microbiological Parameters of the Water of Lake FARIHY MIRAHAVAVY INFERIEUR (Sister Lake) Located in the District of Nosy-Be Region DIANA, Resources and Environment, Vol. 10 No. 2, 2020, pp. 33-40. doi: 10.5923/j.re.20201002.03.
1. Introduction
Nosy-be is the largest island in Madagascar, it is located north-west of Madagascar, it is between 13° 11’ and 13° 30’ south latitude and between 48° 22’and 48° 8’ longitude. It measures 30 km from north to south and 19 km from east to west. This lake is located in the district of DZAMANDZAR. The populations who live around the lake can use it as drinking water, which is why my research will be carried out to analyze the physico-chemical, microbiological and specific treatment parameters corresponding to the results found.
2. Objective of My Research
The first objective of my research is the determination of the concentration for the physical parameters compared with the international standards required for drinking water, respectively for the chemical parameters.The second objective is to determine the levels of microbiological germs to know whether the water ismicrobial or not.The third objective is the specific treatment corresponding to the disinfection of the germs that exist in the water, in order to make the water drinkable without risk of contamination.
3. Research Methods and Materials
For physical parameters: measure directly using a thermometer for temperature measurement, pH meter for pH, measure with turbidimeter for turbidity and measure with conductivity meter for conductivity.For chemical parameters: measurement of total hardness is titrated with CaCO3, calcium, magnesium, sodium, potassium and chloride are measured with the spectrometer apparatus and total iron, lead and aluminum are measured using a PERKIN ELMER lamp and acetylene gas.For the microbiological parameters, we use the colimetry techniques H. Vincent method and the recommended Buttiaux technique to determine the concentration of germs that exist in water using a Smallest Number (MPN).
4. Characteristics of the Lake
It is a sacred lake named FARIHY MIRAHAVAVY (lower and upper sister lake), so the study is done by the lower FARIHY MIRAHAVAVY.
 | Lake Mirahavavy inferior |
5. Quality Standards
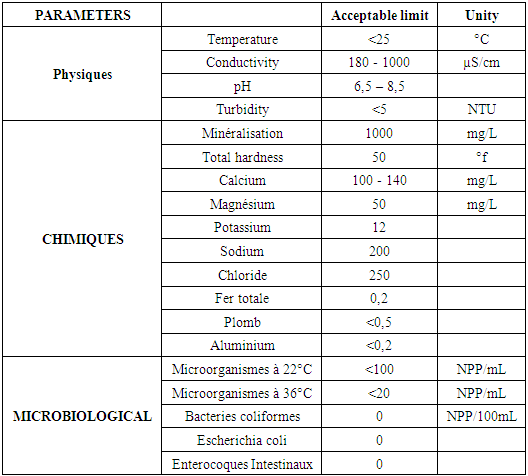
Table 1. Recommendation by the WHO
 |
| |
|
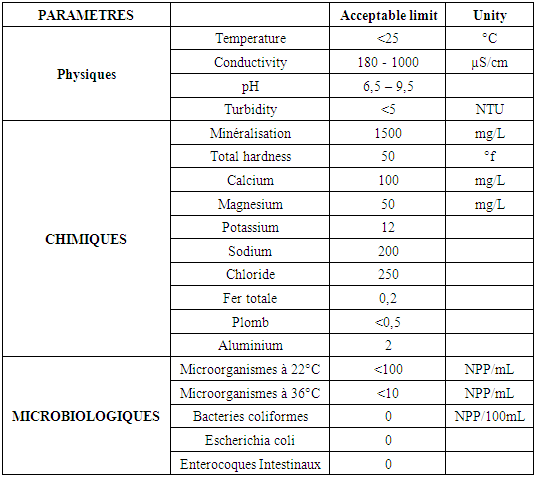
Table 2. Recommendation by the EU
 |
| |
|
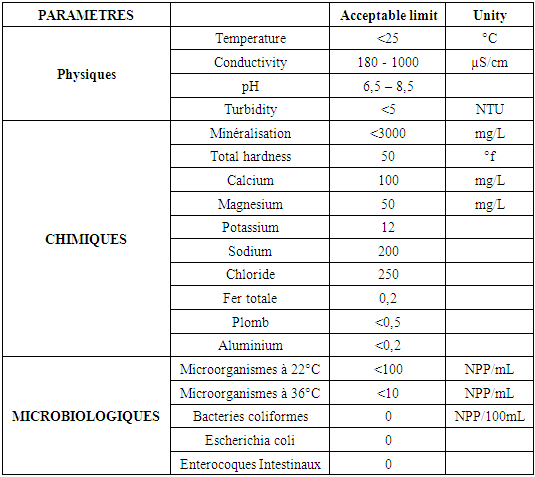
Table 3. Recommendation by the E.M
 |
| |
|
6. Analysis Parameters
Physical parameters:Temperature: determines the natural heat of water, it measures directly with the thermometer.Turbidity: determination of the transparency of the water, possibly the presence of suspended particles.pH: the potential of Hydrogen is the measure of the concentration of H + ions, the water being acidic, basic and neutral.Conductivity: determination of the amount of dissolved matter and dissolved salt in water.Chemical parameters:Total hardness: determination of the calcium and magnesium content in the water.Calcium, magnesium, sodium, chloride and potassium: are abundant and very important elements in water for human consumption.Total iron: obligatory element that exists in water, but their excess in concentration results in the existence of toxicity in water.Lead and Aluminum: Existence in sample indicates water is toxic.Microbiological parameters:The existence of one in the sample of five required for water intended for human consumption indicates the water is microbial.
7. Analysis Results
I - Physical parameters:1 – temperature [3]Table 4. Temperature measurement
 |
| |
|
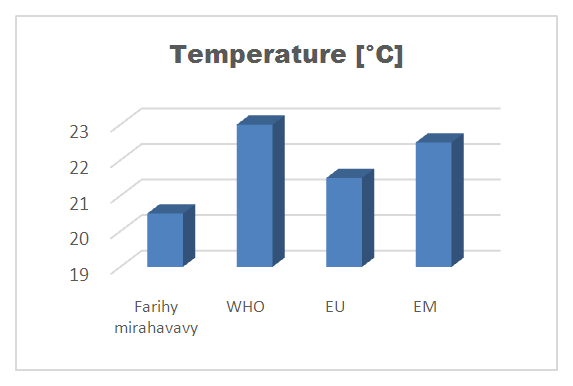 | Figure 1. Temperature measurement |
2 – Conductivity Table 5. Conductivity measurement
 |
| |
|
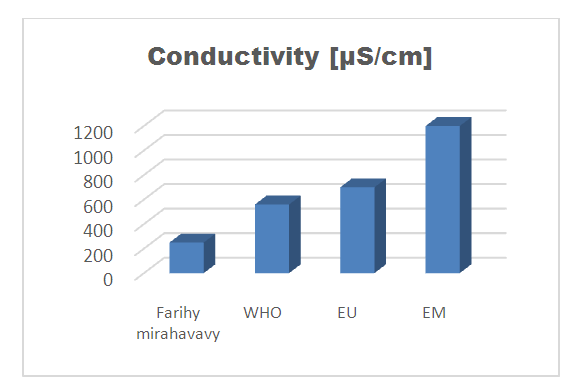 | Figure 2. Conductivity measurement |
3 – pH: [2], [3]Table 6. pH measurement
 |
| |
|
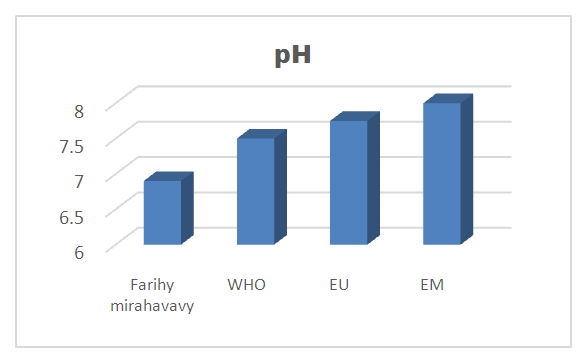 | Figure 3. pH measurement |
4 – Turbidity [1]Table 7. Turbidity measurement
 |
| |
|
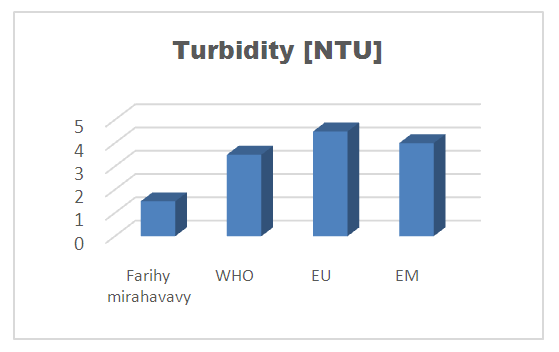 | Figure 4. Turbidity measurement |
II - Chemical parameters1 - MineralizationTable 8. Mineralization concentration
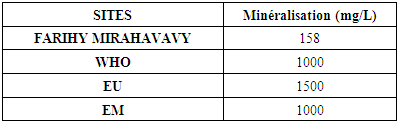 |
| |
|
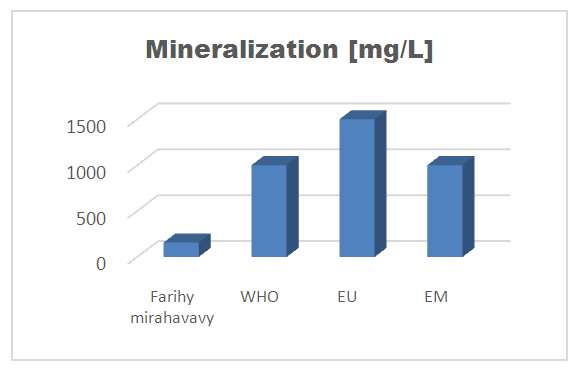 | Figure 5. Mineralization concentration |
2 – Total hardness [4]Table 9. Determination of the total hardness concentration
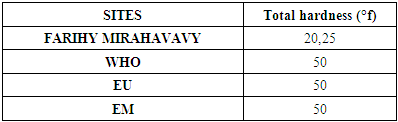 |
| |
|
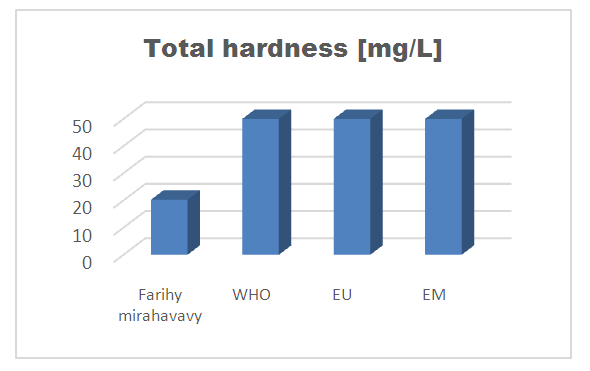 | Figure 6. Total hardness concentration |
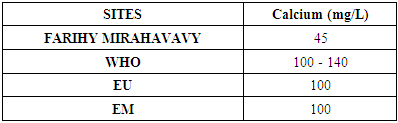
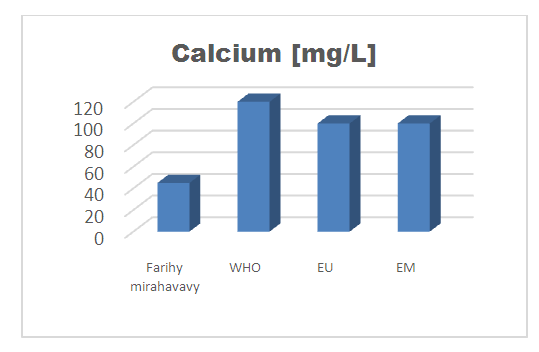
3- Calcium [6]Table 10. Determination of the calcium concentration
 |
| |
|
 | Figure 7. Calcium concentration |
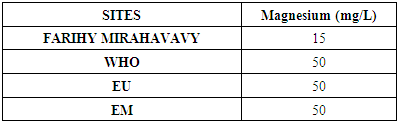
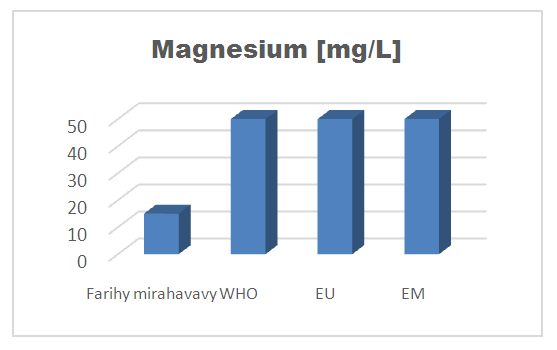
4 – Magnesium [6]Table 11. Determination of the magnesium concentration
 |
| |
|
 | Figure 8. Magnesium concentration |
5 - Potassium [9]Table 12. Determination of the potassium concentration
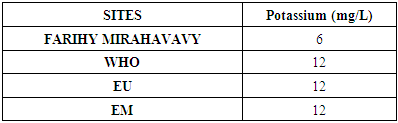 |
| |
|
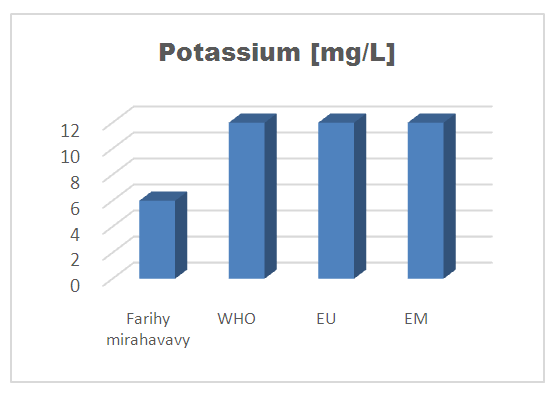 | Figure 9. Potassium concentration |
6 – Sodium [9]Table 13. Determination of the sodium concentration
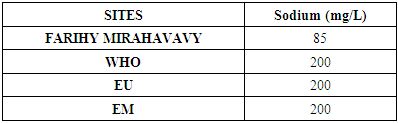 |
| |
|
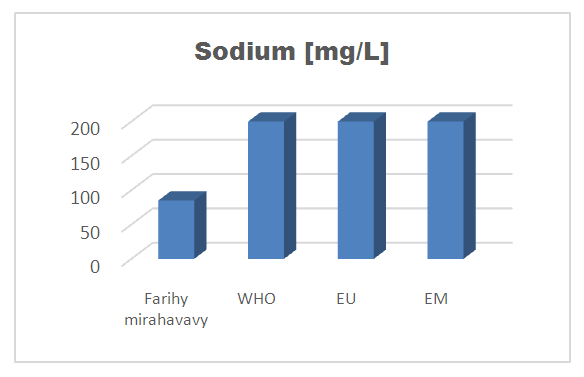 | Figure 10. Sodium concentration |
7 – Chloride [7]Table 14. Determination of the chloride concentration
 |
| |
|
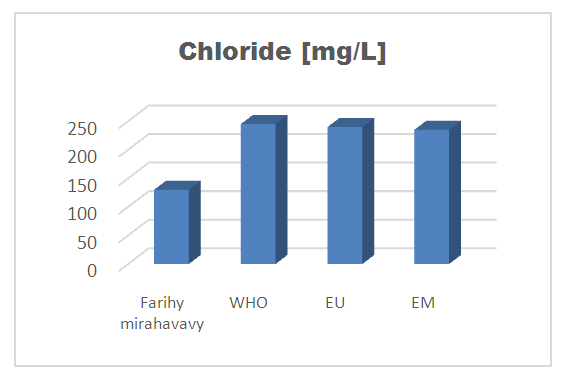 | Figure 11. Chloride concentration |
8 – Total iron [8]Table 15. Determination of the total iron content
 |
| |
|
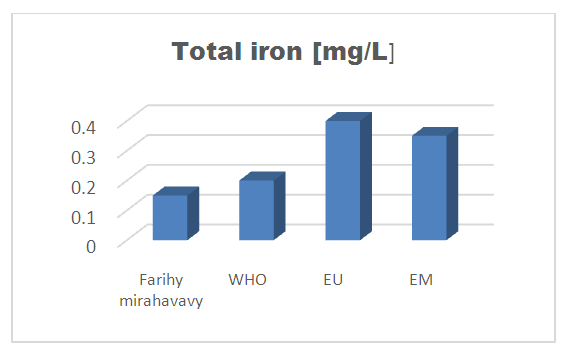 | Figure 12. Total iron concentration |
9 –Lead Table 16. Determination of the lead content
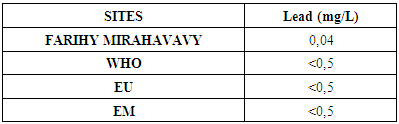 |
| |
|
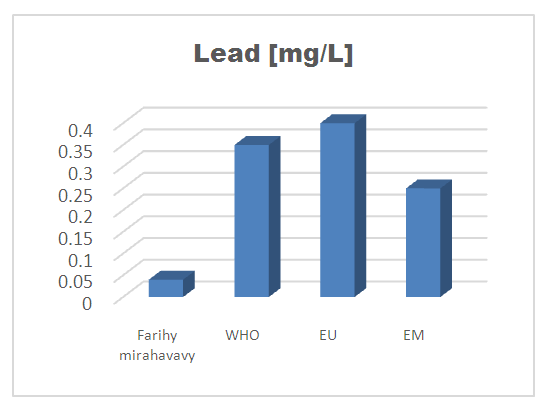 | Figure 13. Lead concentration |
10 – Aluminum [5]Table 17. Determination of the aluminum content
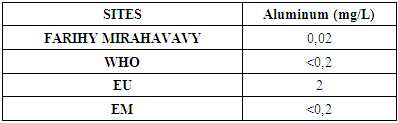 |
| |
|
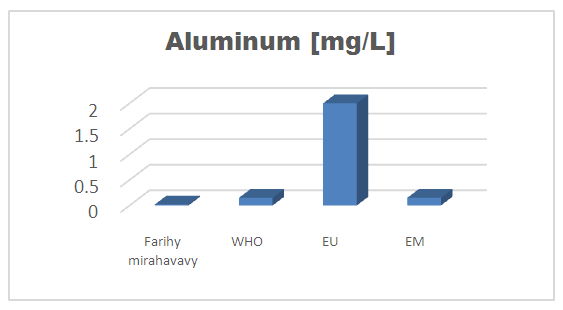 | Figure 14. Aluminum concentration |
III - Microbiological parametersTable 18. Determination of the concentration of microbiological parameters
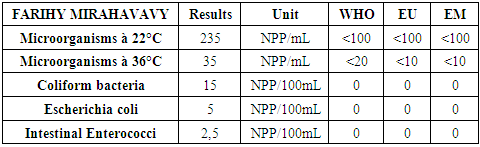 |
| |
|
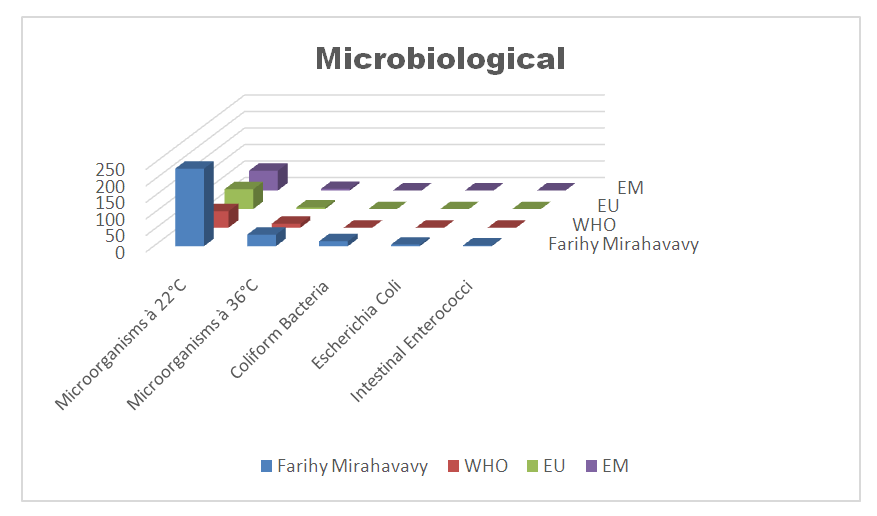 | Figure 15. Concentration of microbiological parameters |
According to this analysis, the values found exceed international standards, therefore the lake water is microbial, it must be treated before use.Treatment method [10]The method used is disinfection by calcium hypochlorite by the "Break-Point" method, ie determining the optimal dose of chlorine for the disinfection of the water in FRIHY MIRAHAVY.The result of the chlorine demand is given in the following table 19. | Table 19 |
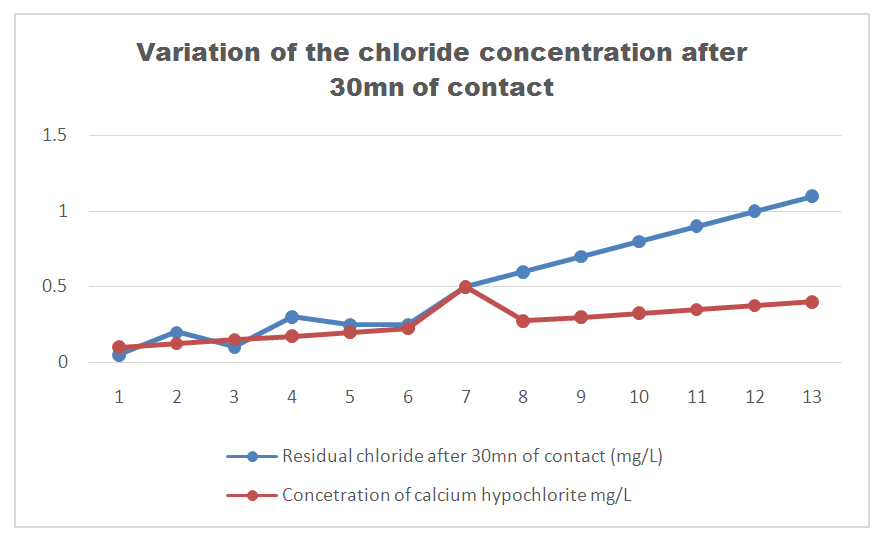 | Figure 16. Variation of the chlorine concentration after 30mn of contact |
From 1 to 3 mg / L: chlorine in the form of amino compounds.From 3 to 4 mg / L: progressive increase in Chlorine.The optimum dose of calcium hypochlorite from the Break-point, the chlorine content of 3.5 mg / L is taken for disinfection.Result immediately after disinfectionTable 20
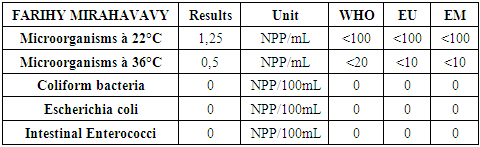 |
| |
|
8. Discussions
For the physical parameters, respectively temperature, pH, turbidity and conductivity, the values found are admissible to the standards required for water intended for human consumption.For the chemical parameters: calcium, magnesium, sodium, potassium and chloride, the concentrations found are acceptable for the international standards required for drinking water, despite their insufficient concentrations such as calcium is 45 mg / L potassium 15 mg / L and sodium is 85 mg / L.For heavy metals such as lead, aluminum and total iron, the concentrations found are very low, so the water is not a risk of contamination, but it is necessary to take the measurement every season and clean the border this lake.For microbiological parameters: the results are very bad, ie the water is microbial, it must be treated before use.I choose the “Break-Point” method for the treatment. According to this method we take the dose 3.5 mg / L of calcium hypochlorite can be taken for disinfection, finally the population uses the water of FARIHY MIRAHAVY without risk of contamination on everything during the rainy season.
9. Conclusions
The water of FARIHY MIRAHAVY is good according to the analysis of the physical parameters, that is to say, the value found is acceptable according to the international standards for drinking water.For the chemical parameters, it is good, despite the insufficient concentration of calcium 45 mg / L, magnesium 15 mg / L and sodium 85 mg / L; The water is drinkable and without risk to health, but lacks the very important elements in water intended for human consumption.Heavy metals Aluminum is 0.02 mg / L and lead is 0.04 mg / L, comparison to international standards require that the limit value less than 0.5 mg / L, for heavy metals; based on this concentration, water is found to be safe for toxicity.For microbiological parameters, water is bad and very dangerous for health. It is necessary to treat by the method of chlorination with the optimal dose of 3.5 mg / L of calcium hypochlorite before being used, in the end the populations who live in the range of FARIHY MIRAHAVY drink this water in complete safety. especially during the rainy season.
References
| [1] | M.FROST et E. HUBER (1997). Mesure en continu de la turbidité, l’Eau l’industrie les nuisances, 206, p 42. |
| [2] | G. VIVIN (février 1957). Mesure et régulation du pH. Génie Chimique, 37. |
| [3] | W. F LANGELIER (1946). Effect of temperature on the pH natural waters J. A. W. W. A. 38, p. 179. |
| [4] | J. RODIER, CH, GRAUDE (1992). Détermination de la dureté dans les eaux par la méthode au complexon III. Bull Institut d’Hydrogène du Maroc, N. S. XII, (3 – 4), p 275. |
| [5] | R. L. BENSON et al. (1990). ON-line determination of residual aluminium in potable and treated waters by flow-injection analysis, Analytica Chimica Acta, 238: 177. |
| [6] | H. DIEHI, J. ELLIGBOE (1956). Indicator for titration of calcium in presence of magnesium using disoudium dihidrogen ethylenediamine tetracetate Anal, Chem, 28, (5), p. 882. |
| [7] | F. E CLARKE (1950). Determination of chloride in water, An Chem, 22, p 553-1458. |
| [8] | A. N. TRIPATHI et al. (1997). Flow injection analysis of iron in rain water with thiocynate and surtactant, Journal of Automatic Chemestry, 19: 45. |
| [9] | J. RODIER, A. POITOUX, C. GRAUDE (1992). Détermination du sodium et du potassium par photométrie de flame. Application au dosage de ces éléments dans le sérum et dans les eaux. (Note technique). Bull, de L’Inst. D’Hygiène du Maroc, N. S., XII, (3 – 4), p 279 – 283. |
| [10] | M. PLISSIER (1981). Etude expérimentale de diverses méthodes d’extraction des virus des eaux. Thèse de Doctorat d’Etat en Biologie Humaine. Université de Montpellier. |





















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

















