-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2020; 10(1): 1-3
doi:10.5923/j.re.20201001.01

Treating Agricultural Soils Contaminated with Cadmium and Copper Using Plant Species
Suaad K. Abd Al-Wahab, Wisam M. Dawood
Department of Biology, College of Education for Pure Sciences, Diyala University, Iraq
Correspondence to: Suaad K. Abd Al-Wahab, Department of Biology, College of Education for Pure Sciences, Diyala University, Iraq.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

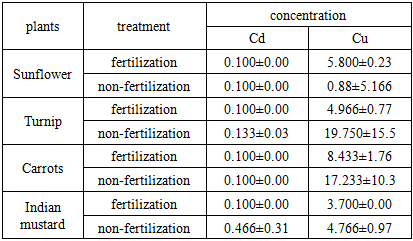
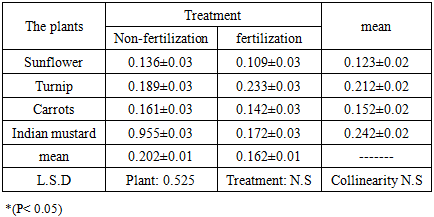
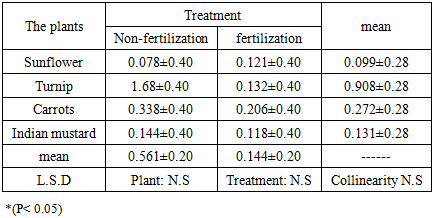
This field study was conducted on four types of plants, namely sun flower Helianthus pumilus L., turnip, Brassica rapa L. carrots Daucus caro L. and Indian mustard Brassica juncea L, to find out the ability of these plants on resisting the toxicity of heavy elements from agricultural soils. The results of the concentration elements of cadmium and copper for the root system showed that the highest value of cadmium was 0.466 mg. Kg-1 in the mustard plant treated without fertilization, whereas that for copper was 19.750 mg. Kg-1 observed in the turnip. As for the bioconcentration factor, the results for the cadmium and copper components were recorded. The highest mean values for the bioaccumulation factor were 0.242 ± 0.02 in the Indian mustard and 0.908 ± 0.028 in the turnip.
Keywords: Cadmium, Copper, Plant specie
Cite this paper: Suaad K. Abd Al-Wahab, Wisam M. Dawood, Treating Agricultural Soils Contaminated with Cadmium and Copper Using Plant Species, Resources and Environment, Vol. 10 No. 1, 2020, pp. 1-3. doi: 10.5923/j.re.20201001.01.
1. Introduction
- Soils can be contaminated with many inorganic chemical compounds which, upon entry to the soil, become part of it and thus affect all forms of life. These inorganic compounds are toxic to humans and animals when they are present in the soil with high concentrations, with their toxicity varying based on the type of toxic element they include [1]. The most important sources of agricultural soil pollution with heavy elements come from fertilizers used to stimulate the life of plants. The use of these elements is incompatible with the global rules and regulations. The pesticides used excessively in agriculture and combustion products include coal and crop residues, as well as the use of part of the silt from treatment plants [2]. Exposure of agricultural soil to pollution with heavy elements and those pollutants they are mixed with causes agricultural soil to lose its fertility and results in the killing of bacteria responsible for the decomposition of the organic materials and stabilizing the soil nitrogen. In addition, plants absorb these elements found in the soil which then reach the human beings through the food chain [3]. The severity of the heavy elements is due to the fact that they are transitional elements with the ability to form fixed complexes with a large group of organic and inorganic compounds present in the bodies of living organisms [4]. The bioconcentration factor (BCF) indicates the efficiency of the plant in withdrawing or extracting heavy elements from the soil and concentrating them in its tissues [5]. It reflects the ratio between the concentration of the element in the root to that in the soil [6], where the higher the value of BCF the more suitable is the plant for extraction [7].
2. Materials and Methods
- Field workThis study was conducted in one of the fields with an area of 400 square meters in the district of Bani Saad, which belongs to the city of Baquba, for the autumn season 2018, where the field was a part of a survey of agricultural lands Concentration of elemental cadmium and copper. The land was divided into 4 sections for planting of 4 types of plants, each section including 6 transactions, 3 for each of the fertilizer and non-fertilization groups. The land of the experiment was plowed in two perpendicular plows by means of a flip-flop plow, smoothed with the use of disc combs, and divided into plates with dimensions of 4 x 5.2 m. The seeds of sunflower were manually planted on 9/7/2018, turnip and carrots on 8/18/2018, and mustard on 7/9/ 2018, by placing 3 seeds in each hole. The land of the experiment was irrigated immediately after planting, with the first three irrigations made in close intervals with a rate of one irrigation every two days, then irrigation continued at a rate of two irrigations per week until the physiological maturity stage. Plants were reduced to a single plant per a hole when they reached a height of 20 cm, and experimental units were grassed whenever needed. The first dose of the liquid fertilizer (Alga seaweed) was added 30 days after planting, while the second dose was added 15 days after the first one.Estimation of elements in the soilAccording to a previous method [8], 1 g of soil samples were taken and 3: 1 ml of nitric acid and hydrochloric acid were added and left for 24 hours after the mixture was placed on a hot plate for 45 minutes. These steps were performed in the Hood Cabin VK-15 Fe. The solution was then transferred to a volumetric flask and the volume was brought up to 100 ml by adding ion free-distilled water. Concentrations of the elements were then measured using an ICP - OES type Ultima 2 JY according to a previous method [9].Estimation of elements in the rootPlant samples were digested according to a previous protocol [10], by taking 0.5 g of crushed samples, adding 10 ml of sulfuric acid for 24 hours, placing the mixture on a hot plat for one hour at a temperature of 150 C, and adding 0.5 ml of perchloric acid with raising the temperature to 250 C for half an hour. These steps were performed in the Hood Cabin type VK-15 Fe, after which the mixture was stransferred to a 50 ml volumetric flask and the volume was completed using ion-free water. The concentrations of cadmium, copper, nickel and lead were measured using an ICP-OES type Ultima 2 JY according to a previously described method [9].Bioconcentration factor The bioconcentration factor is used to determine the amount of heavy element that the plant absorbs from the soil. It is an indicator that shows the ability of the plant to collect the heavy element in its tissues and is calculated using the following equation.
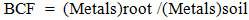 | (1) |
3. Results and Discussion
- Concentration of elemental cadmium and copper in the root systemThe results in table 1 show that the mean concentration of cadmium in the root ranged between (0.466±0.31 - 0.100±0.00) mg. Kg-1, The Indian mustard non-fertilizing recorded a treatment the highest concentration, followed by turnip non-fertilization and then the rest of the plants. The men concentration of copper ranged between (19.750±15.5 - 0.88±5.166) mg. Kg-1, whereas the turnip non-fertilizer recorded the highest concentration and the Sunflower non-fertilizer the lowest concentration. This may be due to the transport of cadmium and copper from the root to the vegetative system, leaving low concentrations in the root.
|
|
|
4. Conclusions
- The Indian mustard plant has the ability to withdraw and accumulate cadmium in the root system by measuring the elements concentration in the root system and calculating the bioconcentration factor.The results indicated that the turnip plant has the ability to withdraw and accumulate copper element in the root system and calculating the bioconcentration factor.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML