Razafitsiferana Theophile, Pr Bruno Razanamparany
University of Antsiranana, Faculty of Sciences Mention Sciences Chimic, Course: Mineral Chemestry, Madagascar
Correspondence to: Razafitsiferana Theophile, University of Antsiranana, Faculty of Sciences Mention Sciences Chimic, Course: Mineral Chemestry, Madagascar.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
For physical parameters such as temperature the value found is 16°C, the required standard is less than 25°C, for turbidity the found value is 1.05 NTU, the limit value is less than 5NTU, for pH the value found is 6.34, the limit value is between 6.5 to 9 and for the conductivity the found value is 33.5 μS / cm, the required value is between 180 to 1000 μS / cm. The physical parameters correspond to 99% for the 0.06 mg / L the limit concentration is required that the water is intended for human consumption. For chemical parameters, such as dissolved oxygen 0.2 mg / L the limit concentration is less than 2mg / L, the salinity level is 0mg / L, the limiting concentration is 0 mg / L, the TAC 0.4°f the limit value is less than 11°f, the nitrate 11.2 mg / L the limit concentration is 50 mg / L, the total hardness 3.2 mg / L the limit concentration is 50 mg / L, Ammonium 0.06 Limit value is 0.5mg / L, Sodium 8.28mg / L limit concentration is 200mg / L, Potassium 11mg / L limit concentration is 12mg / L, Calcium 2.4 limit concentration varies from 100 At 200mg / L, the Magnesium 6.31 mg / L limit concentration is 50mg / L, the Chloride 12.78 mg / L the limit concentration is 250 mg / L and the metals such as Iron, Aluminum, Lead are absent. Therefore the chemical parameters of the water of the AMPOTAKA well are acceptable for the standards required to the drinking water, in spite of the insufficiency of some found concentrations. For microbiological parameters, as microorganisms at 22°C 120 Ufc / mL the required value is less than 100 Ufc / mL, microorganism at 36°C 25 Ufc / mL the required value is less than 20 CFU / mL, Coliforms 70 CFU / 100 mL the limit value is 0, Escherichia Coli 1 Ufc / 100mL, the limit value is 0, Enterococci 1Ufc / 100mL the limit value is 0 and Spores 4 Ufc / 100mL the limit value is 0. Therefore the well water of AMPOTAKA is microbial it must be treated before being used.
Keywords:
Physicochemical, Microbiological parameters and treatment
Cite this paper: Razafitsiferana Theophile, Pr Bruno Razanamparany, Quality Control for the Physicochemical Parameters of the Well Water Located in the AMPOTAKA District in the Hell-Vill Nosy-be Madagascar, Resources and Environment, Vol. 9 No. 4, 2019, pp. 92-98. doi: 10.5923/j.re.20190904.03.
1. Introduction
Nosy be is the largest island of Madagascar, it is located in the North-West of Madagascar. It is located 15 km from the mainland. It measures 30 km from south to north and 19 km from east to west.The district of Nosy Be has six districts, among six district, is called Hell-Vill that is to say in the center of Ville. In this city we have several neighborhoods that exist, so in our search is done in the neighborhood called AMPOTAKA.The population of the neighborhood uses wells as spring water in everyday life, that's why I propose the search for the characteristics of the well water that you used.My research is divided into three parts:- Bibliographic synthesis- Results of analysis of physicochemical and microbiological parameters- Continued treatment of the discussions and we end with the conclusiWell characteristics:The following table 1 shows the characteristics of the AMPOTAKA district well Homographic:The following table 2 gives the number of the population in the district of AMPOTAKA
Homographic:The following table 2 gives the number of the population in the district of AMPOTAKA The numbers of the population in the district of AMPOTAKA to all the towers of 2400 people around.Soil types: soils of volcanic origin.Sedimentation:- The very fine muddy sediments of the bays of mixed origin- Sandy beach sediments with a very coarse texture of clay-loam fraction- The coarse textured sandy sediments contain a small proportion of clay fraction less than 5.5%.Geology and pedology:The island of Nosy Be has a topographic system characterized by an accentuated relief with steep slopes in its eastern part and a gently accentuated relief with a gentle slope in the western part.Hydrography and hydrogeologyThe river systems of the island consist of rivers, lakes and wells.
The numbers of the population in the district of AMPOTAKA to all the towers of 2400 people around.Soil types: soils of volcanic origin.Sedimentation:- The very fine muddy sediments of the bays of mixed origin- Sandy beach sediments with a very coarse texture of clay-loam fraction- The coarse textured sandy sediments contain a small proportion of clay fraction less than 5.5%.Geology and pedology:The island of Nosy Be has a topographic system characterized by an accentuated relief with steep slopes in its eastern part and a gently accentuated relief with a gentle slope in the western part.Hydrography and hydrogeologyThe river systems of the island consist of rivers, lakes and wells.
2. Bibliographic Synthesis
Water intended for human consumption is required by the three standards, WHO, EU and the Malagasy State, the standards are given next to the table of results that we will give below.Analysis ParametersPhysical parametersTemperature: checking the microbe in the waterTurbidity: determining the transparency of the water, regardless of whether the water is cloudy or not.Conductivity: determination of mineral salt levels in waterpH: to know that the water is acidic, basic or neutral.Chemical parametersDissolved oxygen: measurement of organic matter in waterNitrate, Ammonium: measuring the pollution that exists in waterSodium, Potassium, Calcium and Magnesium: are abundant and nutritious elements very important in water.Lead metals like Lead, Aluminum and Iron are toxic, excess concentrations in water is very dangerous to the consumer.Microbiological analysis is very important for water intended for human consumption, ie water that is microbial or not.
3. Measurement Results
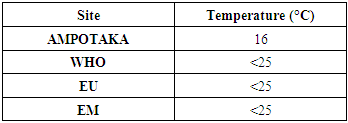
A. Physical parameters1- TemperatureTable 3. Gives the value of the temperature
 |
| |
|
Found value 16°C is acceptable for all three standards because the limit value is 25°C. [4] | Figure 1 |
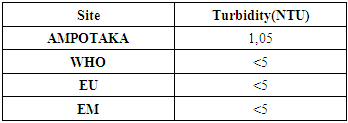
2- TurbidityTable 4. Below gives the measurement results for the turbidity of the well water of AMPOTAKA
 |
| |
|
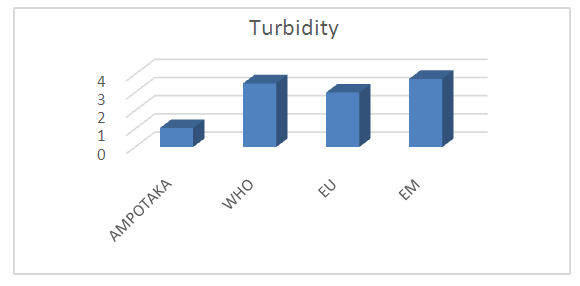 | Figure 2. Measurement of turbidity. [1] |
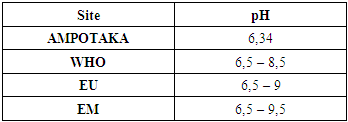
According to this value found 1.05 NTU, the water is clear, it is acceptable.3- The pHTable 5. below gives the pH value
 |
| |
|
The pH value found is 6.34, so just around the limit value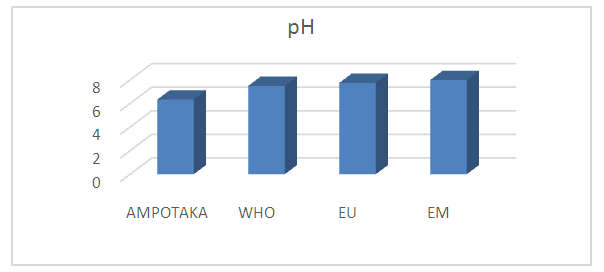 | Figure 3. pH measurement. [4], [5] |
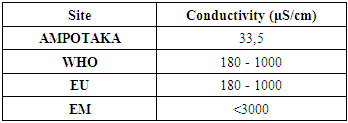
4- ConductivityTable 6. Below gives the measurement result for conductivity
 |
| |
|
The found value 33.5 µS / cm is outside the required value, so the water is low in organic matter.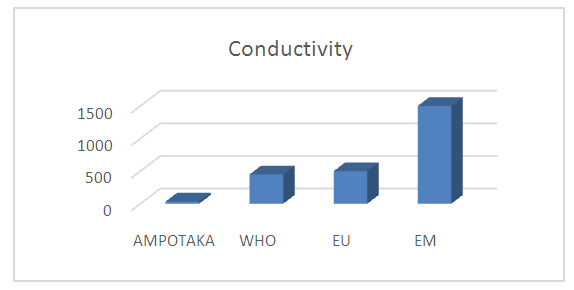 | Figure 4. Measurement of conductivity. [3] |
B- Chemical parameters1- Dissolved oxygenTable 7. Below gives the measurement of the concentration of dissolved oxygen
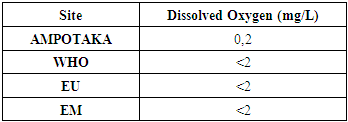 |
| |
|
The concentration found is 0.2 mg / L, low in organic matter, so the water is good.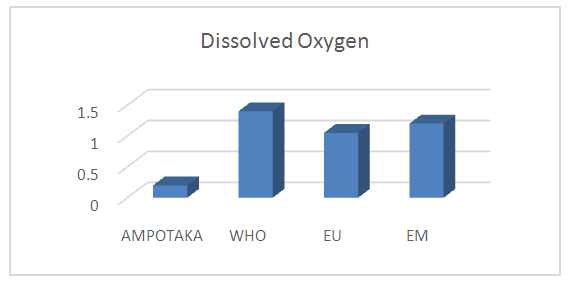 | Figure 5. Measurement of dissolved oxygen concentration. [2] |
2- T.A.CTable 8. below gives the value of the TAC
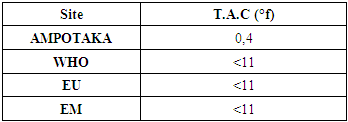 |
| |
|
The value found is 0.4°f, the water is low in basic salt content which means that the water is good.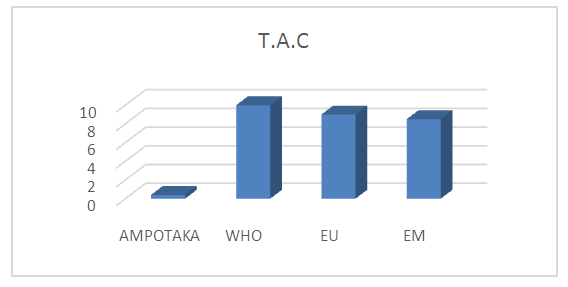 | Figure 6. Measurement of the concentration in T.A.C. [6] |
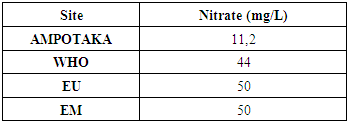
3- NitrateTable 9. below gives the concentration of nitrate in the water of the AMPOTAKA well
 |
| |
|
The concentration found is 11.2 mg / L, compared to the limit concentration 50 mg / L, the rate of pollution in water is low, so it is good.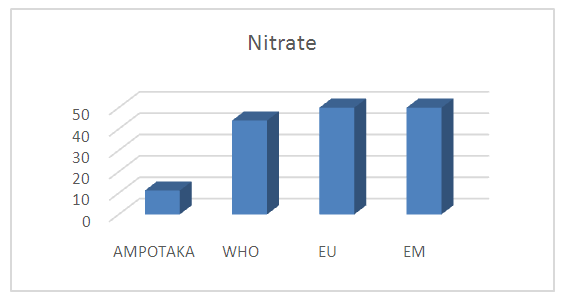 | Figure 7. Measurement of nitrate concentration |
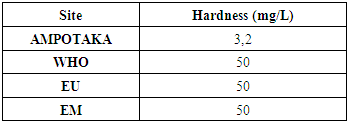
4- HardnessThe following table 10 gives the concentration
 |
| |
|
The concentration found 3.2 mg / L the water is not hard compared to the limiting concentration.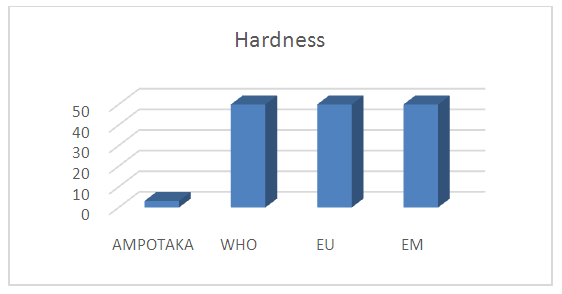 | Figure 8. Measurement of the concentration in total hardness. [7] |
5 - AmmoniumTable 11. Below gives the ammonium concentration
 |
| |
|
The concentration found is 0.06 mg / L the pollution in the water is very low, it is acceptable for drinking water.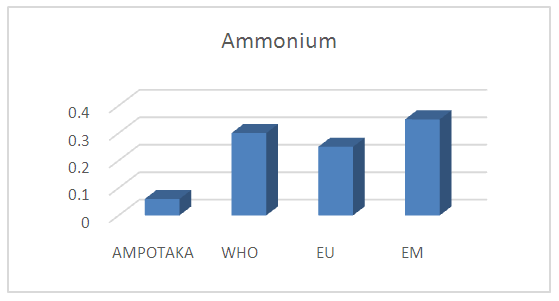 | Figure 9. Measurement of Ammonium Concentration. [8] |
6 - SodiumTable 12. below gives the measurement of the sodium concentration
 |
| |
|
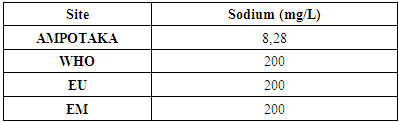
The concentration found is 8.28 mg / L, it is very low by the required standards.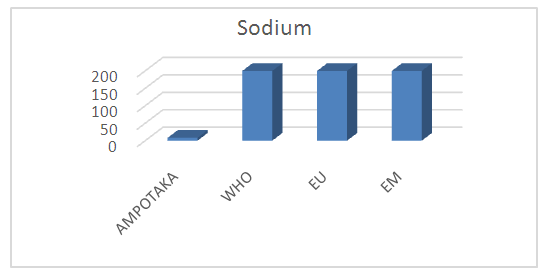 | Figure 10. Sodium concentration measurement. [12] |
7 - PotassiumThe following table 13 gives the measurement of the potassium concentration
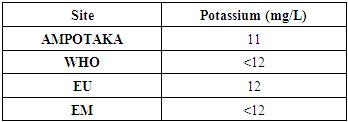 |
| |
|
The water is rich in potassium because the concentration found is 11 mg / L.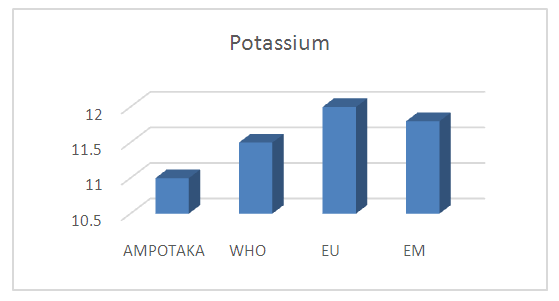 | Figure 11. Measurement of Potassium Concentration. [12] |
8 - CalciumTable 14. Below shows the measurement of calcium concentration
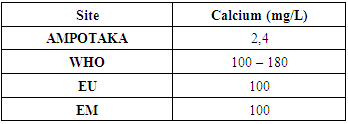 |
| |
|
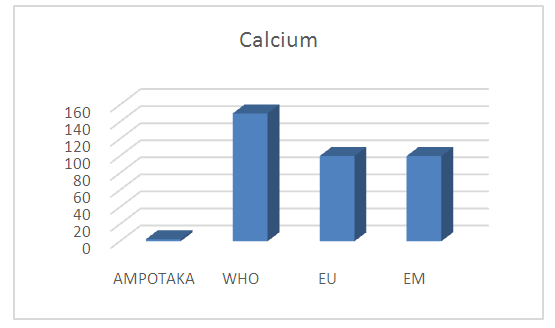 | Figure 12. Calcium concentration measurement The concentration found 2.4 mg / L isverylowcompared to the required standards, so the water lacks calcium. [9], [11] |
9 - MagnesiumThe following table gives the measurement of the magnesium concentration
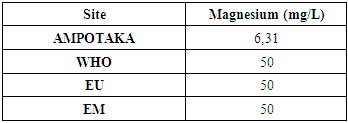 |
| |
|
The concentration foundis 6.31mg / L, compared to the limited consultation itisinsufficient. | Figure 13. Measurement of Magnesium Concentration [11] |
10 - ChlorideTable 16. Below gives the chloride concentration
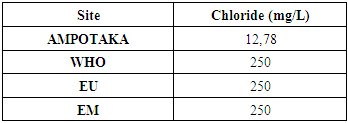 |
| |
|
The concentration of chlorine in the water is low compared to the limit concentration for drinking water. | Figure 14. Measurement of chlorine concentration |
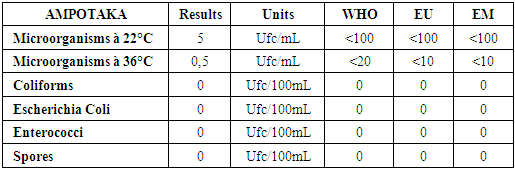
Note:For heavy metals such as Lead, Aluminum and Iron do not exist in the sample. [10]C- Microbiological parametersTable 17. Below gives the measurement of the concentration of microbiological parameters in the sample
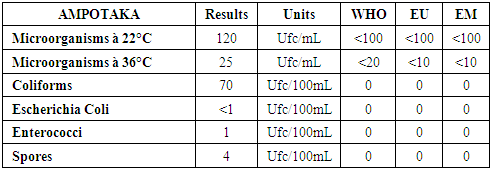 |
| |
|
According to these results the water is microbial, it must be treated before being used. [13]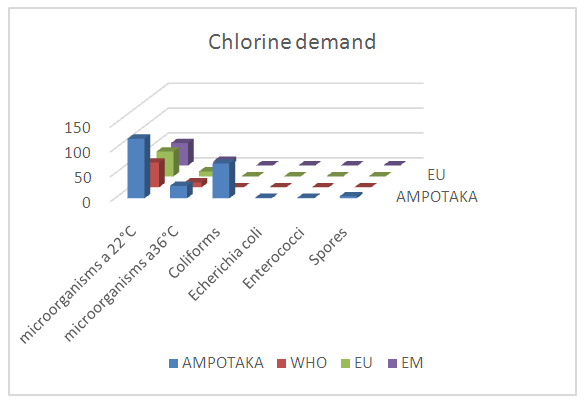 | Figure 15. Measurement results for microbiological parameters |
TreatmentI propose the treatment for disinfection; the demand for chlorine.The demand for chlorine consists in adding to the same volume of water increasing doses of calcium hypochlorite. The residual chlorine level measured after a given time as a function of the added dose passes through a minimum called Breack-Point before increasing regularly.Reagents:- Calcium Hypochlorite 1g / L (1mL contains 1mg hypochlorite)- orthotolidineMaterials- 6 containers (1L, 500 mL or 230 mL beakers)- Pipets of 1 mL or 2 mL- Hydrocure Comparator- Free chlorine blister 0.1 to 2 mg / LOperating mode:In a series of 6 containers of a given volume. Introduce V mL of water to be disinfected.- Add in each of them with a pipette increasing amounts of calcium hypochlorite 1g / L- Shake and cover each container with a sheet of paper. Leave in contact 30mn.- Shake in the middle and at the end of the experiment.- Measure the residual chlorine in the 6 BeakersExpressions of the resultsLet - di: the dose of hypochlorite in each beaker of volume V (in g / L)- Vi: the volume of hypochlorite to add (in mL)Vi = (di.V) / 1000Results of the testsTable 18. Below gives the demand for chlorine
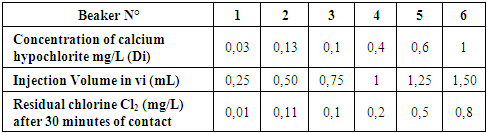 |
| |
|
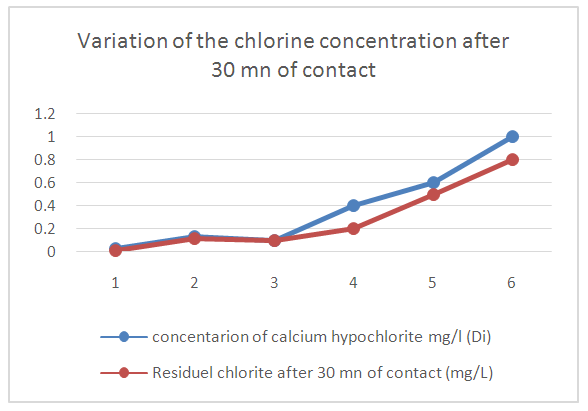 | Figure 16. Variation of the chlorine concentration after 30 mn of contact (Break Point) |
Curve for Representation Demand for chlorineAccording to this curve the dose of calcium hypochlorite is taken from 3,5 mg / L for disinfection.Results just after treatmentTable 19. Below gives the measurement result of the disinfection just after the treatment
 |
| |
|
Results interpretation- Physical parametersThe physical parameters of the well water of the AMPOTAKA district are eligible for the standards required for water intended for human consumption.- Chemical parametersThe chemical parameters of the well water in the AMPOTAKA district meet the limit concentrations for water intended for human consumption, despite the insufficiency of some concentration at the calcium level of only 2.4 mg / L but the concentration Limit ranges from 100 mg / L to 180 mg / L, sodium 8.28 mg / L also the limit concentration is 200 mg / L and the magnesium 6.31 mg / L limit concentration is 50mg / L.For heavy metals such as lead, aluminum and iron are absent in the sample.- MicrobiologicalThe six microbiological parameters required for the drinking water are not verifiable, all results obtained are excluded at the limit value, as microorganisms at 22°C 120 Ufc / mL against the limit value is less than 100 cfu / mL, Escherichia Coli 70 Ufc / 100mL the limit value is 0 and the Enterococci 1 Ufc / 100mL the limit value is 0.For the treatment we use the chlorine demand for the disinfection of the well water of the AMPOTAKA district with a precaution the dose of 3mg / L of calcium hypochlorite for the disinfection.
4. Conclusions
The well water of the AMPOTAKA district is good in terms of physical parameters and chemical parameters, despite the concentration are low for some parameters like calcium, magnesium and sodium.For the microbiological parameters the water is not drinkable because the result shows that the water is microbial, so it is necessary to treat before being used.I propose the method for disinfection in dosing of calcium hypochlorite by the Breack Point curve ie the increase of the curve from the 3,5 mg / L that you used for the disinfection, finally that the population AMPOTAKA district drink safely and safely even in the rainy season.
References
| [1] | B. WELTE et A. MONTIEL (2002). Etude comparative de mesure de la turbidité sur différentes eaux. L’Eau l’industrie les nuisances, 255. p. 125. |
| [2] | L. W. WINKLER (1888). The determination of dissolvedoxygen in water. Ber, 21. P. 2843. |
| [3] | Organisation Internationale de Métrologie Légale (1981). Standard solutions reproduccing the conductivity of electrolytes international recommendation, n°56, 1st ed. Bureau International de Métrologie Légale, Paris, France. |
| [4] | W. F. LANGELIER (1946). Effect of temperature on the pH naturel waters J.A.W.W.A. 38. P. 179. |
| [5] | G. VIVN (février 1957). Mesure et régulation du pH. Génie Chimique. 37. |
| [6] | T.E. LARSON and L.M. HENLEY (1955). Determination of lowalkalinity or acidity in water. Anal. Chem. 27. 851. |
| [7] | J. RODIER. CH. GRAUDE (1952). Détermination de la dureté dans les eaux par la méthode au complexon III. Bull. Institut d’Hygiène du Maroc. N.S. XII, (3-4), p. 275. |
| [8] | R.L. BOOTH and R.F. THOMAS (1973). Selectiveelectrodedetermination of ammonia in water and wastes. Environ. Sci. Technol. 7: 523. |
| [9] | T.J. CARDWELL et al. (1988). Determination of calcium in waters, milk and wine by discontinuous-flow analysis, Analyst. 115: 1235. |
| [10] | F.E. CLARKE (1950). Determination of chloride in water, An Chem, 22, p. 553-1458. |
| [11] | S. MOTELLIER et al. (2000). Quantitative capillaryelectrophoreticanalysis for calcium and magnesium in sodium-matrix waters, Analytica Chimica Acta, 410: 11. |
| [12] | J. RODIER, A. POITOUX, C. GRAUDE (1952). Détermination du sodium et du potassium par photométrie de flamme. Application au dosage de ces éléments dans le sérum et dans les eaux. Bull. De l’Inst, d’Hygiène du Maroc, N.S, XII, (3-4), p. 279-283. |
| [13] | P. VILAGINESS.B. SARRETTE, R. VILAGINESS (1988). Détection en continu du poliovirus dans les eaux de distribution publique. C.R. Acad, Sci. Paris, 307, p. 1983-1988. |


 Homographic:The following table 2 gives the number of the population in the district of AMPOTAKA
Homographic:The following table 2 gives the number of the population in the district of AMPOTAKA The numbers of the population in the district of AMPOTAKA to all the towers of 2400 people around.Soil types: soils of volcanic origin.Sedimentation:- The very fine muddy sediments of the bays of mixed origin- Sandy beach sediments with a very coarse texture of clay-loam fraction- The coarse textured sandy sediments contain a small proportion of clay fraction less than 5.5%.Geology and pedology:The island of Nosy Be has a topographic system characterized by an accentuated relief with steep slopes in its eastern part and a gently accentuated relief with a gentle slope in the western part.Hydrography and hydrogeologyThe river systems of the island consist of rivers, lakes and wells.
The numbers of the population in the district of AMPOTAKA to all the towers of 2400 people around.Soil types: soils of volcanic origin.Sedimentation:- The very fine muddy sediments of the bays of mixed origin- Sandy beach sediments with a very coarse texture of clay-loam fraction- The coarse textured sandy sediments contain a small proportion of clay fraction less than 5.5%.Geology and pedology:The island of Nosy Be has a topographic system characterized by an accentuated relief with steep slopes in its eastern part and a gently accentuated relief with a gentle slope in the western part.Hydrography and hydrogeologyThe river systems of the island consist of rivers, lakes and wells.















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML















