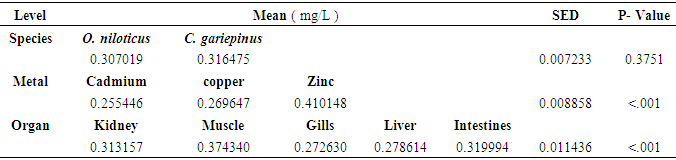
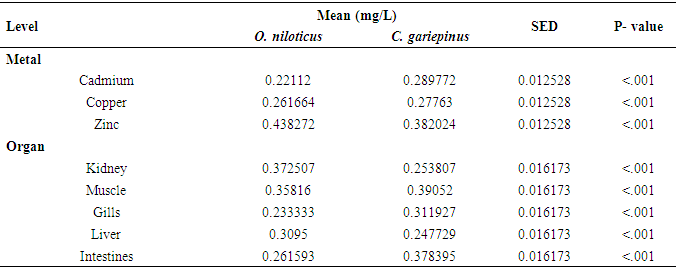
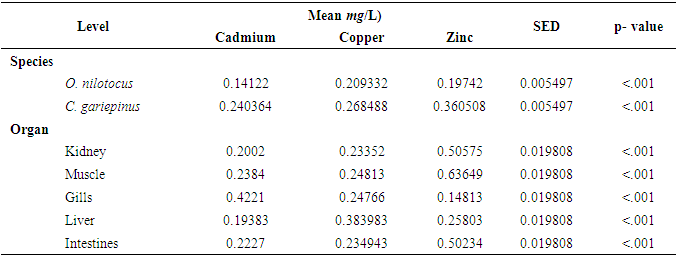
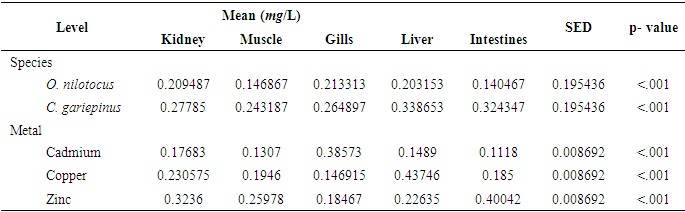
| [1] | Bahnasawy, M., Khidr, A. and Dheina, N. (2009) Seasonal Variations of Heavy Metals from Concentrations in Mullet, Mugil Cephalus and Liza Ramada (Mugilidae Lake Manzala, Egypt). Journal of Applied Sciences Research, 5 (7): pp. 845-852. |
| [2] | Garcia, J.C., Martinez, D.S.T., Alves, O.L., Leonardo, A.F.G., Barbieri E. (2015) Ecotoxicological effects of carbofuran and oxidized multiwalled carbon nanotubes on the freshwater fish Nile tilapia: Nanotubes enhance pesticide ecotoxicity. Ecotoxicol. Environ. Saf., 111: 131-137. |
| [3] | MacFarlane, G. B., Burchettt, M. D., (2000). Cellular distribution of Cu, Pb, and Zn in the Grey Mangrove Avicemnia marina (Forsk.). Vierh Aquatic Botanic, 68: 45–59. |
| [4] | Kumar, B., Sajwan, K. S. and Mukherjee, D. P. 2012. Distribution of Heavy Metals in Valuable Coastal Fishes from North East Coast of India Turkish Journal of Fisheries and Aquatic Sciences 12: 81-88. |
| [5] | Censi, P., Spoto, S. E., Saiano, F., Sprovieri, M., Mazzola, S., Nardone, G., Di Geronimo, S. I., Punturo, R., Ottonello, D. (2006). Heavy metals in coastal water systems. A case study from the northwestern Gulf of Thailand. Chemosphere, 64: pp 1167–1176. |
| [6] | Shrivastava, V. S. (2011). Study for Determination of Heavy Metals in Fish Species of the River Yamuna (Delhi) by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES). Advances in Applied Science Research, 2:161-166. |
| [7] | Muiruri, J., Nyambaka, H., & Nawiri, M. (2013). Heavy metals in Water and Tilapia Fish from Athi-Galana-Sabaki Tributaries, Nairobi. International Food Research Journal, 23:891-896. |
| [8] | Camusso, M., Vigano, L., Baitstrini, R., (1995). Bioaccumulation of trace metals in rainbow trout. Ecotox. Environ. Safe., 31: pp 133–141. |
| [9] | Dsikowitzky, L., Mengesha, M., Dadebo, E., Eduardo, C., Calvalho, V. d., & Sindern, S. (2012). Assessment of heavy metals in water samples and in fish tissues of edible fish species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environmental Monitoring and Assessment. |
| [10] | Namminga, H. N., Wilhm, J., (1976). Effects of high discharge and an oil refinery cleanup operation bon heavy metals in water and sediments in Skeleton Creek. Proceedings of the Oklahoma Academy of Science, 56: 133–138. |
| [11] | Storelli, M. M., Storelli, A., D’ddabbo, R., Marano, C., Bruno, R., Marcotrigiano, G. O., (2005). Trace elements in loggerhead turtles (Carettacaretta) from the eastern Mediterranean Sea: Overview and evaluation. Environ. Pollut., 135: pp. 163–170. |
| [12] | Özmen, H., Külahçı, F., Çukurovalı, A., and Doğru, M., (2004). Concentrations of heavy metal and radioactivity in surface water and sediment of Hazarlake (Elazığ, Turkey). Chemosphere, 55: pp. 401–408. |
| [13] | Begum, A., HariKrishna, S., Khan, I. (2009), Analysis of Heavy metals in Water, Sediments and Fish samples of Madivala Lakes of Bangalore, Karnataka: International Journal of ChemTech Research, 1(2), pp. 245-249. |
| [14] | Fernandes, C., Fontaínhas-Fernandes, A., Cabral, D., Salgado, M. A., (2008). Heavy metals in water, sediment and tissues of Liza saliens from Esmoriz–Paramos lagoon, Portugal. Environ. Monit. Assess. 136: pp. 267-275. |
| [15] | Pote, J., Haller, L., Loizeau, J.L., Bravo, A.G., Sastre, V., and Wildi, W., (2008). Effects of a sewage treatment plant outlet pipe extension on the distribution of contaminants in the sediments of the Bay of Vidy, Lake Geneva, Switzerland. Bioresource Technol., 99: pp. 7122–7131. |
| [16] | Praveena, S. M., Radojevic, M., Abdullah, M. H., Aris, A. Z., (2008). Application of sediment quality guidelines in the assessment of mangrove surface sediment in Mengkabonglagoon, Sabah, Malaysia. Iran. J. Environ. Health. Sci. Eng., 5 (1): 35-42. |
| [17] | Öztürk, M., Özözen, G., Minareci, O. and Minareci, E. 2009. Determination of heavy metals in fish, water and sediments of Avsar Dam Lake in turkey. Iran. Journal of Environment, Health. Science and Engineering 6 (2): pp. 73-80. |
| [18] | Ismaniza Ismail & Idaliza Mat (2012). Analysis of heavy metal in water and fish (tilapia species) samples from TasikMutiaraPuchogi. The Malysian Journal of analytical Sciences, pp 346-352. |
| [19] | Mahboob S, Al-Balawi HFA, Al-Misned F, Al-Quraishy S, Ahmad Z (2014) Tissue metal distribution and risk assessment for important fish species from Saudi Arabia. Bull Environ Contam Toxicol 92: 61-66. |
| [20] | Rashed M.N., (2001). Cadmium and Lead levels in fish (Tilapia nilotica) tissues as biological indicator of Lake water pollution pp. 75-89. |
| [21] | Ogoyi D. O; Mwita C.J; Nguu E.K; Shiundu P.M (2011). Determination of heavy metal content in water, sediments and microalgae from Lake Victoria. The open Environmental Engineering Journal , 156-161. |
| [22] | Nweeze N. O., Mahmood L. B., and Aisha U. I (2014): Lithological Studies and Aigal diversity, the useful fool for assessment of fish pond water quality,” Asian Academic Research Journal of Mullidisplinatin Auline. |
| [23] | Omima A.S.A. Aboud (2010): Impact of pollution with lead, mercury and cadmium on the immune response of Oreochromis niloticus. New York Science Journal. 3(9) pp. 12-16. |
| [24] | Saleh, YS, Marie M-A.S. (2014) Assessment of metal contamination in water, sediment, and tissues of Arius thalassinus fish from the Red Sea coast of Yemen and the potential human risk assessment. Environ. Sci. Pollut. Res., DOI 10.1007/s11356-014-3780-0. |
| [25] | Canli, M., Atlı, G., (2003). The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut., 121: pp 129– 136. |
| [26] | Barhoumi S., Messaoudi I., Gagne F. and Kerkeni A. 2012. Spatial and seasonal variability of some biomarkers in Salaria basilisca (Pisces: Blennidae): Implication for biomonitoring in Tunisian coasts, Ecological Indicators, 14 (1): 222-228. |
| [27] | Tüzen, M., (2003). Determination of heavy metals in fish samples of the MidDamLakeeBlack Sea (Turkey) by graphite furnace atomic absorption spectrometry. Food Chemistry, 80: pp.119–123. |
| [28] | Karthikeyan, S., Palaniappan, P.L. R.M., and Sabhanayakan, (2007). Influence of pH and water hardness upon accumulation in edible fish, Cirrhusmrigala. J. Environ. Biol., 28: pp.484-492. |
| [29] | Authman M.M.N, Abbas W.T, Gaafar A.Y (2012) Metals concentrations in Nile tilapia Oreochromis niloticus (Linnaeus, 1758) from illegal fish farm in Al-Minufiya Province, Egypt, and their effects on some tissues structures. Ecotoxicol Environ Saf 84: 163-172. |
| [30] | Heath, A.G. (1991): Pollution and fish Physiology. Lewis Publishers, Boca Raton, Florida, USA. |
| [31] | Nussey, G., VanVuren, J.H.J., and Du Preez H.H., (2000) Bioaccumulation of Chromium, Manganese, Nickel and Lead in the Tissues of the Moggel, Labeoumbrata (Cyprinidae) from Witbank Dam, Mpumalanga. Water SA., 26: pp. 269-289. |
| [32] | Bolormaa, O., Baasansuren, J., Kawasaki, W.M., and T. Hattori. (2006): Analysis of heavy metals in water samples from a mining area in Mongolia. J. of Nuclear Instr. andMethods in Physics Res., 243: pp. 161-166. |
| [33] | Yoon, Seokjoo, Sang-Seop Han and Rana, S.V.S., (2008) Molecular markers of heavy metal toxicity – A new paradigm for health risk assessment. J. Environ. Biol., 29: pp.1-14. |
| [34] | Maier D, Blaha L, Giesy JP, Henneberg A, Köhler HR, et.al. (2014) Biological plausibility as a tool to associate analytical data for micropollutants and effect potentials in wastewater, surface water, and sediments with effects in fishes. Water Research (2014). |
| [35] | Hilmy, A.M., El Domiaty, N.A., Daabees, A.Y., and Moussa, F.I., (1987): Short-term Effects of Mercury on Survival, Behavior, Bioaccumulation and Ionic Pattern in the Catfish (Clariaslazera). Comp. Biochem. Physiol., 87, 303-308. |
| [36] | Olojo, E., Olurin, K.B., Mbaka, G., and Oluwemimo, A.D, (2005). Histopathology of the gills and liver tissues of the African catfish (Clariasgariepinus) exposed to Lead. African J. Biotech., 4: pp. 117-122. |
| [37] | Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol. Environ. Saf., 113: 145-151. |
| [38] | Adeyeye, E.I., Akinyugha, R.J., Febosi, M.E., and Tenabe, V.O. (1996) Determination of some metals in Clariasgariepinus (Cuvier and Valenciemes), Cyprinuscarpio (L) and Oreochromisniloticus (L) fishes in a polyculture fresh water pond and their environment. Aquacult., 47, pp 205-214. |
| [39] | Srivastava, R., and Srivastava, N., (2008) Changes in nutritive value of fish Channapuntustus after chronic exposure to Zinc. J. Environ.Biol., 29: 299-302. |
| [40] | Bauvais C, Zirah S, Piette L, Chaspoul F, Coulon I.D (2015) Sponging up metals: Bacteria associated with the marine sponge Spongia officinalis. Mar. Environ. Res., 104: 20-30. |
| [41] | Raburu, P.O. (2003). Water quality and the status of aquatic macroorganisms and ichthyofauna in River Nyando, Kenya. PhD thesis, Moi University, Kenya. |
| [42] | APHA (1998).Starndard methods for the examination of water and wastewater. 20th Ed. Aprill, W. and Sims, R. -Evaluation of the use of prairie grasses for stimulating polycyclic aromatic hydrocarbon treatment in soil. Chemosphere, 4. (1990). 20 (1-2): p. 253-265. |
| [43] | Ochumba, P.B.O. (2005). Periodic massive fish kills in the Kenyan part of Lake Victoria. Water Quality Bulletin. 112: pp. 119–22, 130. |
| [44] | Kersten, M. and Forstner, U. (1987): Cadmium in the Aquatic Environment, Nriagu J.O and Sprague J.B, ed. NY, NY: Wiley, pp. 51-88. |
| [45] | Mwashote, B. (2003). Levels of Cadmium and Lead in water, sediments and selected fish species in Mombasa, Kenya. Western Indian Ocean Journal, 25-34. |
| [46] | Javed., M., & Usman, N. (2011). Accumulation of heavy metals in fish: A human health concern. International Journal of Environmental Sciences. 2: pp 23-25. |
| [47] | Milan C., Maina, M., Onyia, H. U., L. Ozoemena, P.E, (2012). Heavy metal pollution in benthic fishes from Kiri Dam in Guyuk local goverment area of Adamawa State, Nigeria. African Journal of Biotechnology, pp. 11755-11759. |
| [48] | Tuionen., T., Pihlstrom., M., Arvola., L., & Rask, M. (2006). Concentration of heavy metals in food web components of small boreal lakes. Boreal Environmental Research, pp. 183-194. |
| [49] | Njogu., P., J.M. Keriko., R.N. Wanjau., & J.J. kitetu. (2011). Distribution of heavy metals in various lake matrices: water, soil, fish and sediments: A case study of the Lake Naivasha basin, Kenya. Jomo Kenyatta University of Ariculture Science and Technology, pp. 23-47. |
| [50] | Forstner, U. and Wittmann, G.T.W. (1981). Metal Pollution on the Aquatic Environment, Spring-Verlag, Berlin, Heidelberg, New York. pp. 486. |
| [51] | Ekpo, K. E., Asia, I. O., Amayo, K. O. and Jegede, D. A. (2008). Determination of lead, cadmium and mercury in surrounding water and organs of some species of fish from Ikpoba River in Benin City, Nigeria. Int. J. Phy. Sci. 3 (11), pp. 289-292. |
| [52] | Edem, C.A., Osabor, V., Iniama, G., Etiuma, R., and Eke, J. (2009) Distribution of Heavy Metals in Bones, Gills, Livers and Muscles of (Tilapia) Oreochromisniloticus from Henshaw Town Beach Market in Calabar Nigeria. Pak. J. Nutr. 8 (8): pp. 1209-1211. |
| [53] | Yilmaz, F. (2009). The Comparison of Heavy Metal Concentrations (Cd, Cu, Mn, Pb, and Zn) in Tissues of Three Economically Important Fish (Anguilla) anguilla, Mugilcephalus and Oreochromisniloticus) Inhabiting Kˆycegiz Lake-Mugla (Turkey). Turkish Journal of Science & Technology, 4 (1): pp. 7-15. |
| [54] | Ali, A. N. (2007). Ecological Study on Some Water Characteristics Used in Fish Farms and their Relation to Fish Productivity. Ph. D. Thesis. Al-Azhar Unive. Faculty of Science, Department of Chemistry, Egypt. pp. 44. |
| [55] | Onyia L. U., Millam C. and Ese D. S. (2007). Investigation of heavy metals in four commercial fishes along Upper River Benue, Yola.Inter. J. Physical Sci. 2 (2), pp: 35-36. |
| [56] | Bols N, Brubacher J, Ganassin R and Lee L. (2001). Ecotoxicology and inert immunity in fish. Dev. Comp. Immunol, 25(8), 853-873. |
| [57] | Karadele-Akin H and Unlu E. (2007). Heavy metal concentration in water, sediments and some benthic organisms from Tigris river. Turkey Environ. Monit Assess, 131: pp. 323-337 http://dx.doi.org/10.1007/s10661-006-9478-0. |
| [58] | Chettopadhyay BA, Chettorjee A & Mukhopadhyay SK. (2002). Bioaccumulation of metals in east Calcuta wetland Ecosystem. Aquat. Ecosys. Healthmanag, 5 (2), pp: 191-203. |
| [59] | Yasser A. Gh. and Naser M. D. (2011): Impact of pollutants on fish collected from different parts Shatt AI- Arab River: a histopathological study. Environmental Monitoring and Assessment. 181: 175-182. |
| [60] | El-Nemr AL. (2003) Concentration of certain heavy metals in imported frozen fish in Egypt. J. Aqua. Biol. Fish, 7, 139-154. |
| [61] | Khaled A. (2004). Heavy metal concentrations in certaintissues of five commercially important fishes from El-MexssBay, Al-Exandria, pp.1-11. |
| [62] | WHO (2004). Copper in Drinking Water. Background Document for the Development of WHO Guidelines for Drinking Water Quality. Geneva: WHO pp. 19. |
| [63] | Lalah., J., E.Z.Ochieng., & S.O.Wandiga. (2008). Sources of heavy metal input into WinamGulf, Kenya. Environmental Contamination and toxicology, pp. 277-284. |
| [64] | Hellawell, J.M. Ed., 1986. Biological Indicators of Freshwater Pollution and Environmental Management. Elsevier Applied Science Publishers Ltd., London and New York, pp.546. |
| [65] | Olaifa, F. G., Olaifa, A. K. and Onwude, T. E. (2004). Lethal and Sublethal Effects of Copper to the African Catfish (Clariasgariepinus). African J. Biomed.Research 7: 65-70. |
| [66] | Al-Kahtani, M. A. (2009). Accumulation of heavy metal in Tilapia fish (Oreochromisniloticus) from Al-Khadoud Spring Al-Hassa, Saudi Arabia. American Journal of Applied Sciences, pp 2024-2029. |
| [67] | Rauf A, Javed M & Ubaidullah M. (2009). Heavy metal levels in three major Carps (Catlacatla, Laberorohita and Cirrhinamrigala) from the river Ravi, Pakistan. Pakistan Vet. J, 29 (1) pp. 24-26. |


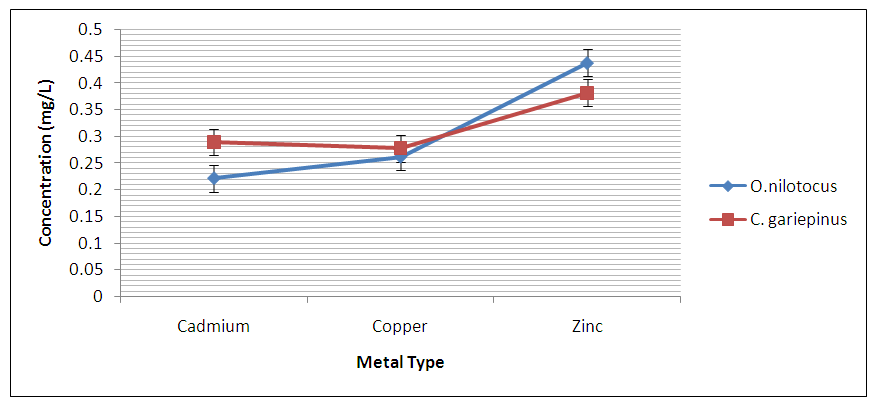
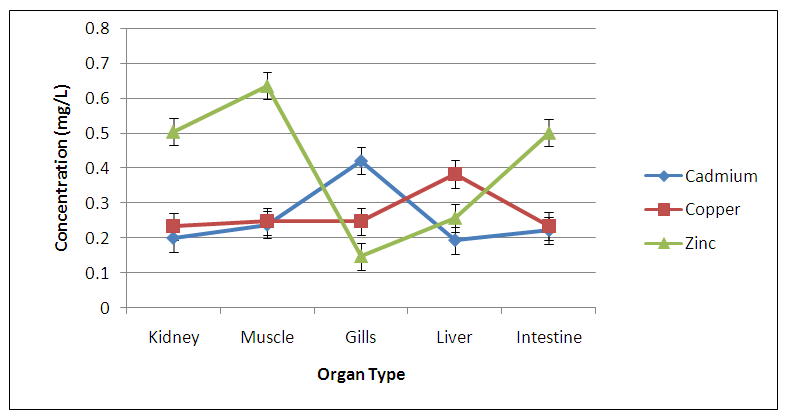
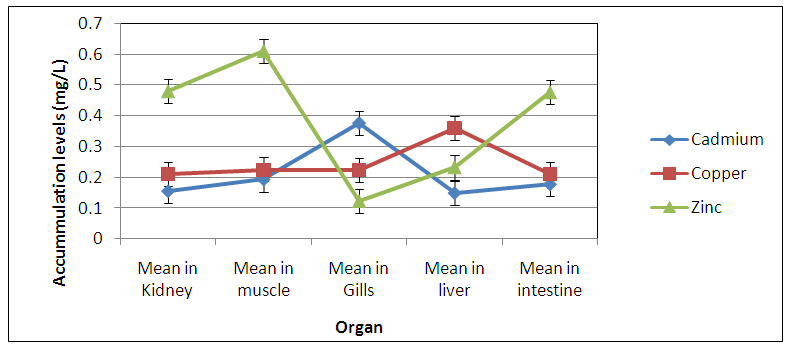
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML