-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2019; 9(3): 49-57
doi:10.5923/j.re.20190903.01

Chemical Retention Function of Thaulla Area of Small Reservoir; A Case Study in Ulankulama Tank, Anuradhapura, Sri Lanka
K. G. M. J. W. Gunapala, N. S. Abeysingha
Department of Agricultural Engineering and Soil Science, Faculty of Agriculture, Rajarata University of Sri Lanka, Puliyankulama, Anuradhapura, Sri Lanka
Correspondence to: N. S. Abeysingha, Department of Agricultural Engineering and Soil Science, Faculty of Agriculture, Rajarata University of Sri Lanka, Puliyankulama, Anuradhapura, Sri Lanka.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Thaulla is a term used to identify the upper peripheral region of small reservoirs /tanks located in Sri Lanka. This study was carried out to investigate the chemical retention function of the thaulla area of Ulankulama tank in North Central province of Sri Lanka. 13 soil and 15 tank water quality parameters were tested for this purpose. Soil samples were collected from 19 points in the thaulla area. Water samples were collected three times from the tank during the dry period. All soil and water samples were tested using standard methods. The results indicated that the thaulla area of the Ulankulama tank acts approximately as a wetland. It is evident by high accumulation of Fe (1819.65 ppm) and Al (1586.75 ppm) with a considerable retention of P (74.80 ppm), Ca (163.79 ppm) and Mg (203.67 ppm) in tested soil samples. This function was further corroborated with the observations of higher concentrations of P, Fe, and Al in the samples, which were higher than reference values of the Reddish Brown Earth soil found in the area. This phenomenon is supported by the evidence of low concentrations of Fe (1.40 ppm), Al (0.009 ppm) and P (0.095 ppm) in tank water during dry spell. N was found to be the limited nutrient in the thaulla area (0.073%) and it was identical to the wetlands. This study showed evidence for the chemical retention function of thaulla and suggests the restoring of the thaulla area of Dry Zone tanks in Sri Lanka.
Keywords: Chemical retention, Thaulla, Ulankulama tank, Wetland
Cite this paper: K. G. M. J. W. Gunapala, N. S. Abeysingha, Chemical Retention Function of Thaulla Area of Small Reservoir; A Case Study in Ulankulama Tank, Anuradhapura, Sri Lanka, Resources and Environment, Vol. 9 No. 3, 2019, pp. 49-57. doi: 10.5923/j.re.20190903.01.
Article Outline
1. Introduction
- The dry zone of Sri Lanka receives rainfall within a limited period of the year. In order to overcome the limited water availability for agriculture, the ancient communities developed their own water management system known as the Tank Cascade System (TCS). A cascade is a connected series of tanks organized within a meso catchment of the dry zone landscape, storing, conveying and utilizing water from an ephemeral rivulet (Madduma Bandara, 1985). Small manmade water storing structures, commonly known as ‘wewa’ is the main element of TCS, which mainly supplies water for paddy cultivation. Water is continuously recycled in TCS and excess water from the upstream tank is captured in the downstream tanks. It supplies a water flow from upper tank to the lower tank through cultivated lands. The TCS of Sri Lanka are extensively considered as one of the unique water conveying and management systems among the ancient civilizations of the world and has been recognized as a Globally Important Agricultural Heritage Systems” (GIAHS) by the FAO (FAO, 2018).The area situated just above the tank bed called as Wew thaulla through which runoff water enters into the tank. It covers with numerous types of plants and creepers. This micro-land region is partly covered with water and completely flooded during the rainy season (Abeysingha et al., 2018). Thaulla is a breeding and feeding ground for birds and also provides food for animals. This forest cover with waterloving trees, termed as gassgommana (upstream tree plantations) (Mahatantila et al., 2007) is a part of thaula. It acts as a wind barrier and reduces the occurrence of waves in the tank and evaporation of water. In addition, during high flood period, this area provides different habitats for fish and other aquatic species. It is hypothesized that the plants in the upper periphery of the tank may act as plants in constructed wetland and remove excessive nutrients, which drains to the tank (Mahatantila et al., 2010). At present the thaulla area of most of the tanks has been degraded, the land cover has changed and the intended function has been neglected. In addition, due to increased population pressure, thaulla areas have been increasingly encroached during the last decades (Bebermeier et al., 2017). This area and the adjacent reservation catchment were strictly protected by ancient farmers (Mahatantila et al., 2008). At present, it is very often not considered as one of the most important bio-engineering structure in TCS. Therefore, there is an urgent need to restore the tank thaulla area of all tanks in Sri Lanka. One way of understanding the importance of thaulla is scientifically convince its role. Therefore, Abeysingha et al. (2016), conducted a preliminary study to test the soil quality of thaulla area of Ulankulama tank analyzing only few parameters. The present study was extended to investigate the chemical retention function of thaulla area by taking thaulla area of Ulankulama tank in the Anuradhapura district as a case study so that researchers and policy makers can understand better the role of the thaulla area of TCS in Sri Lanka.
2. Methodology
2.1. Study Site
- Ulankulama tank is situated in Thirappane Divisional Secretariat Division of the Anuradhapura district in Sri Lanka. It is located about 20 km south of Anuradhapura city, adjacent to the Anuradhapura - Maradankadawala - Kekirawa road. Ulankulama tank is located in the middle part of Ulagalla tank cascade (Fig. 1). The total average annual rainfall in the area is about 1,445 mm and the area receives more water from North East monsoon. The major soil group is reddish brown earth (Alfisols) comprising sandy-loam to sandy clay loam in texture. Gross area and net catchment area of the tank are 1523 ha and 904 ha, respectively. Tank capacity is 460,000 m3 and water spread area at spill is 44.7 ha (Navaratne and Gunawardena, 1999). Farmers cultivate about 41 ha of paddy in the command area using the water of the Ulankulama tank. This tank has prominent thaulla area of about 0.43 km2 (Fig. 1).
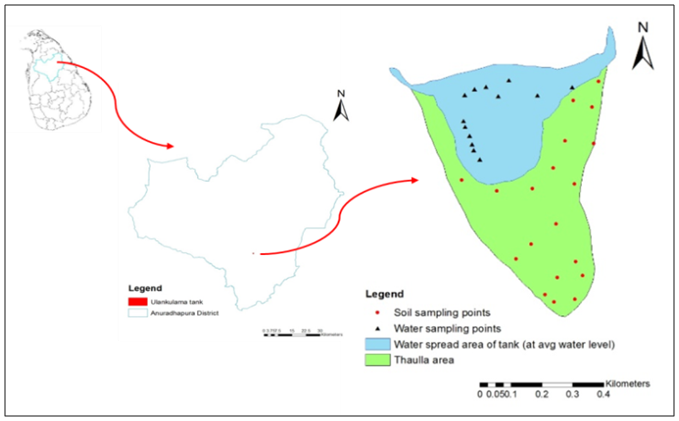 | Figure 1. Ulankulama tank in Anuradhapura district in Sri Lanka |
2.2. Soil Sampling and Analysis
- Soil samples were collected from 19 randomly selected points in thaulla of Ulankulama tank to represent the entire thaulla area, from two depths as 0-15 cm and 15-30 cm using stainless steel soil augers at the beginning of the study. Global Positioning System (GPS) locations of sampling points in thaulla area were also recorded. The soil samples were analyzed for various physicochemical parameters i.e. pH, Electrical Conductivity (EC), total N, available P, total K, Na, Ca, Mg, Fe, Al, As, Cd and Pb contents in the laboratory by using standard laboratory methods. pH and EC were determined using the multi-parameter analyzer (HATCH, Sension 156) with the 1: 2.5 soil-water suspension method and 1: 5 soil-water suspension method respectively. Total N content of soil samples was determined by the Kjeldahl method (Bremmer and Muloaney, 1982). Available P content of soil samples was determined by UV- visible spectrophotometer with Olsen method (Olsen et al., 1954). Total K, Na, Ca, Mg, Fe, Al, As, Cd and Pb contents were measured using the Inductivity Couple Plasma Optical Emission Spectrophotometer (ICP-OES) procedure after acid digestion of soil samples (Martin et al., 1994).
2.3. Water Sampling and Analysis
- Gilma is a folkloristic term used to denote a water collected area of a tank during a dry spell. Water samples were collected from the gilma area of Ulankulama tank during dry spell. Sampling was limited to three times (during October 2016, December 2017 and March 2017) due to drought condition during the dry spell. Sampling points were randomly selected to represent the entire water spread area and water spread areas varied during sampling periods. GPS locations of selected water sampling points in Ulankulama tank were recorded. Water samples were collected in a pre-clean sampling bottles in the early hours of the day.The collected water samples were tested for physicochemical parameters such as water temperature, pH, EC, and Total Dissolved Solids (TDS), immediately using the multi-parameter analyzer (HATCH, Sension 156). Other parameters such as ammonium nitrogen (NH4+ - N), nitrate nitrogen (NO3- - N), available P, total K, Na, Ca, Mg, Fe, Al, As, Cd and Pb were analyzed in the laboratory by using standard laboratory methods. Calibration of instruments was carried out before measurements using standard methods. NH4+ - N content of water samples was determined by UV – visible spectrophotometer with 4500 NH3 F Phenate method (Solorzano, 1969). NO3- - N content of water samples was tested by UV – visible spectrophotometer with salicylic acid method (Cataldo et al., 1975). Available P content of water samples was determined by UV- visible spectrophotometer with ascorbic acid method (Olsen et al., 1954). Total K, Na, Ca, Mg, Fe, Al, As, Cd and Pb contents were measured by ICP-OES following the method of Martin et al. (1994).
2.4. Statistical Analysis and Interpolation of Data
- The descriptive statistics were calculated for both the soil and the water of the thaulla area and water spread area of the tank, respectively using SPSS software. Point sample values of pH, EC, N, P, K, Na, Ca, Mg, Fe, Al, As, Cd and Pb contents of soil samples were interpolated to the thaulla area using the Kriging method of interpolation in Arc GIS 10.2 software.
3. Results and Discussion
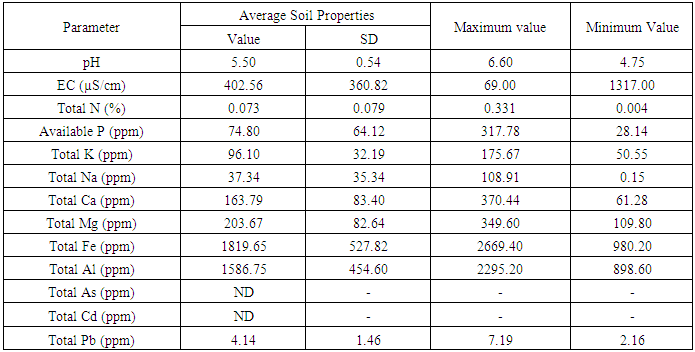
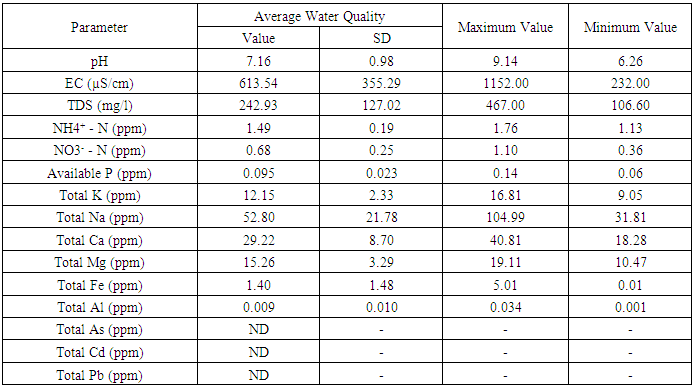
- Average soil properties at average depth (0-30 cm soil) of the thaulla area of Ulankulama tank are shown in table 1 with their standard deviation and maximum and minimum values while table 2 shows the average results of the physicochemical analysis carried out for water samples collected from gilma area of the Ulankulama tank during dry spell between September 2016 and April 2017.
|
|
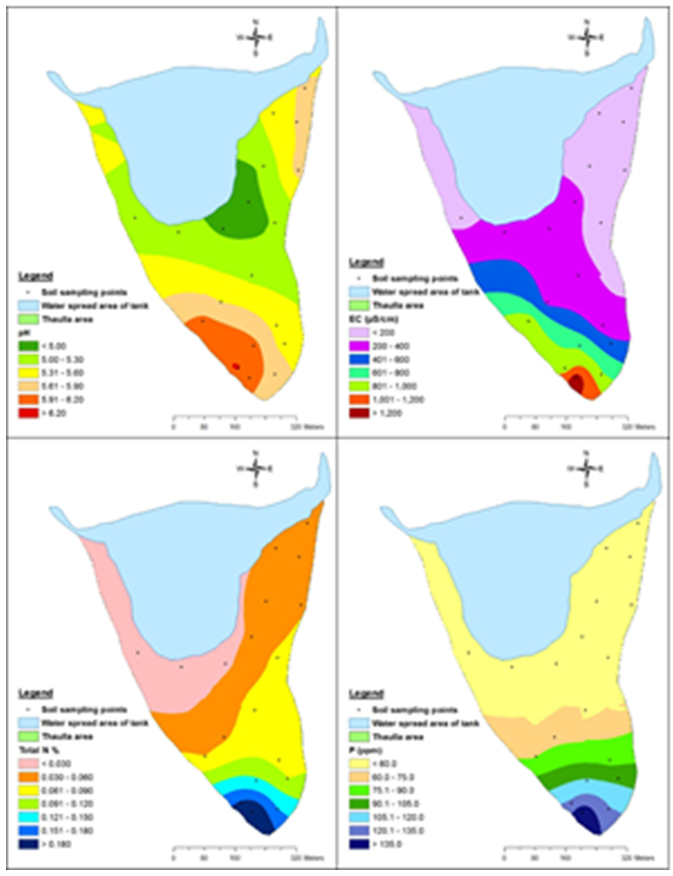 | Figure 2. Variation of average pH, EC, total N and available P (0–30 cm soil) of thaulla area of Ulankulama tank |
3.1. Soil pH of thaulla Area and Water pH at Low Water
- pH affects the chemical reaction between water and soil minerals and pH determines the solubility and biological availability of some elements. The soil pH of the thaulla area at average depth (0-30 cm) ranged from 4.6 to 7.3 with an average of 5.5. It indicates that the soil condition of thaulla area varied from strongly acidic to slightly basic conditions. As an average (5.5), the soils of the area is slightly acidic in nature. The average pH (5.75) of 15-30 cm soil depth is higher than the average pH (5.25) of 0-15 cm soil depth indicating that surface soils were acidic than the subsurface soils (15-30 cm depth). Considering the interpolated pH in the thaulla area, there was no clear trend in the variation of pH of thaulla area (Fig. 2). pH of the tank water at low water level ranged from slightly acidic (6.26) to strongly basic (9.14) and the average pH value of tank water during the study period was 7.16. The average pH value of tank water during low water level (7.16) was higher than the average pH of soils at thaulla area (5.5).
3.2. Variation of Electrical Conductivity (EC) of Soil and Water
- Total nitrogen is the sum of nitrate, nitrite, organic nitrogen and ammonia nitrogen. The Total Kjeldahl Nitrogen (TKN) is only organic nitrogen and ammonia nitrogen. TKN was determined as total The EC of the soil in thaulla area at average depth (0-30 cm) during the sampling time ranged from 69 to 1317 µS/cm with an average of 402.56 µS/cm. It indicates that the EC of thaulla area varied from non-saline to strongly saline conditions. The average EC of tank water during dry spell (613.54 µS/cm) exceeded the average EC of thaulla area but tank water and soil of thaulla area were in moderately saline conditions when considering the average EC values. EC of Reddish Brown Earths (RBE) and Low Humic Gley (LHG) soil associations in the dry zone of Sri Lanka ranged from 0.3 to 0.6 dS/cm (30 – 60 µS/cm) (Vidyarathna et al., 2008). The average EC of soil in thaulla area at Ulankulama tank highly exceeded this range and this may be a result of higher dissolution of ions in water when water flows through the thaulla area. The average EC of 0-15 cm soil depth (516.36 µS/cm) is higher than the average EC of 15-30 cm soil depth (288.75 µS/cm) indicating that surface soils of thaulla area absorb more salts. Moreover, there was a decreasing trend in EC in 0-15, 15-30 cm and average depth (0-30 cm) (Fig. 2) towards the water spread area of the tank, especially though southwestern direction as the main water flow is from this direction. Abeysingha et al. (2016) also observed the same trend in EC in this thaulla. The reason may partly be the dilution effect of water closer to the water spread areas and the absorption of electrolyte by the plants in the thaulla area.
3.3. Total Nitrogen (N) in Soil in thaulla Area and Nitrogen (N) in Water
- Total nitrogen is the sum of nitrate, nitrite, organic nitrogen and ammonia nitrogen. The Total Kjeldahl Nitrogen (TKN) is only organic nitrogen and ammonia nitrogen. TKN was determined as total nitrogen in this study. The observed values for total N of the thaulla area at average depth (0-30 cm) varied from 0.004% to 0.331% with average total N of 0.073%. The amount of prevailing total N extent for RBE and LHG soils are 11.6% and 23.22%, respectively (Nandasena, 2002). However, observed values of total N in thaulla area were lower than those values. Abeysingha et al., 2016 also observed the lower N concentration in same thaulla area. Nitrogen was shown to be the most limiting nutrient in wetlands and other aquatic systems. In wetlands, during the flooded period, anthropogenic nitrogen input is released from soil to the overlying water in the form of ammonia (Reddy and D’Angeolo, 1997). Similarly, when thaulla area is flooded during the heavy rainy period, nitrogen may release to the water from the soil. Moreover, total N in depth 0-30 cm (Fig. 2) was in a decreasing trend towards the water spread area of the tank. This decreasing trend can also be attributed to the releasing of nitrogen to the water by the soil processes where more water is available closer to the water spread areas. It has been identified that major processes responsible for nitrogen removal in wetlands are denitrification (Tanner, 2004), uptake by plants, and subsequent nitrogen accumulation in the plant biomass (Borin and Toccheto, 2007), sedimentation (Borin and Toccheto, 2007), and volatilization (Vymazal, 2007). A similar process can happen in the thaulla area as there is growth of aquatic plants and grasses in the thaulla area, especially towards the water spread area of the tank, which may account for the loss of nitrogen. Ammonium nitrogen concentration of the tank water during the dry spell ranged from 1.13 to 1.76 mg/l with an average of 1.49 mg/l. The nitrate-nitrogen concentration of the Ulankulama tank during the study period ranged from 0.36 to 1.10 mg/l with 0.68 mg/l of average nitrate concentration. The nitrate-nitrogen values observed in the waters of the tank at dry spell indicated lower values than permissible level of 10 mg/l for drinking (WHO, 1984). Nitrate-nitrogen in water may be absorbed by aquatic plants in the thaulla area and the water spread area of the tank. Wijesundara et al. (2012) showed that nitrate-nitrogen concentration of water at Thirappane, Alisthana, and Meegasagama tanks of the Anuradhapura district in Sri Lanka varied from 2.43 to 4.11 mg/l during the dry season (July, August, and September). Though these tanks are closer to the Ulankulama tank nitrate-nitrogen range is higher than that of Ulankulama tank during the dry period. The reasons may be the less prominent and unhealthy thaulla areas of those tanks when compared to the thaulla area of Ulankulama tank.
3.4. Available Phosphorous (P) Content in Soil in thaulla Area
- Available P in soil is considered to be a fairly good measure of P supplying capacity of a soil. During the sampling time, available P concentration of the thaulla area at average depth (0-30 cm) varied from 28.14 to 317.78 ppm with average available P concentration of 74.80 ppm. The available P concentration of RBE soils in Anuradhapura district varied from 1.6 to 20 mg/kg (Karunadasa and Duminda, 2013). The average available P concentration of the thaulla area of Ulankulama tank exceeded this range and much higher concentration of P (1254ppm) was reported by Abeysingha et al. (2016) during 2015. These observations infer that there is P accumulation in the thaulla area. Just above the thaulla area and the upstream catchment area consists of paddy fields and chena lands, which can release a heavy load of P containing residues of chemical fertilizers through runoff during the rainy season leading to higher P concentration in the thaulla area of Ulankulama tank. The average available P of 0-15 cm soil depth (89.24 ppm) is higher than the average available P of 15-30 cm soil depth (60.36 ppm). It may indicate the adsorption of P and the accumulation of P in the upper layers of soil. Available P in average depth 0-30 cm (Fig. 2) is decreasing towards the water spread area of the tank, which is similar to the behavior of total nitrogen. Unlike nitrogen, phosphorous cannot be lost from wetlands through metabolic process thus phosphorus tends to accumulate in the system (Reddy and D’Angeolo, 1997). Thus, the accumulation of phosphorous in thaulla area resembles the similar function of a wetland. Further, the average available P concentration of tank water during dry spell (0.095 ppm) was lower than the available P concentration of thaulla area. It indicates that the thaulla area is functioning as a sink for P thus, reducing the overall P concentration in the tank water. This study tested the average texture of soil at the depth of 0 – 30 cm of thaulla area, and observed sand, silt and clay content as 32.95, 40.53 and 26.52 % respectively. Silt content is comparably higher than those of sand and clay. One reasons for higher concentration of P observed in thaulla area may be due to the P combine with the higher content of silt. Moreover, P can be retained by oxides and hydroxides of Fe and Al when the soil is acidic (Fisher and Acreman, 2004). It can be precipitated with Ca and Mg when the soil is basic (Reddy et al., 2000). Hence, the medium acidic soils (average pH - 5.5) of and observed higher average Fe and Al concentrations (1819.65 and 1586.75 ppm) along thaulla area may be the reason for P retention in thaulla area. Also, the observed higher average Ca and Mg concentrations of thaulla area (163.79 and 203.67 ppm) suggest P precipitation with Ca and Mg in areas where pH is higher. This P retention with Fe, Al, and Ca and Mg further illustrate the function of thaulla similar to a wetland. Available phosphorous concentration of the water at tank varied from 0.065 to 0.136 mg/l during the dry period and average available phosphorous concentration was 0.095 mg/l. These values recorded in low water level is very much lower than the soil P values.
3.5. Total Potassium (K) in Water and thaulla Soil
- The average potassium concentration of Ulankulama tank water was 12.15 mg/l during the dry spell and it ranged from 9.05 to 16.81 mg/l. In general, surface water in Sri Lanka is rich in Na+, Ca2+, and Mg2+ but low in K+ concentration (Dissanayake et al., 1982). The concentration of potassium, nitrate nitrogen and phosphate in tanks of the Thirappane cascade showed a bimodal pattern, which was coincided with the bimodal rainfall of the dry zone (Wijesundara et al., 2013). Their concentrations in surface water were higher in the dry season while relatively low during the wet season. Potassium fertilizers applied in the paddy field at the upstream catchment areas and the accumulated cow dung and other organic residues in the thaulla area may account for elevated concentration of potassium in water of the Ulankulama tank. The soil potassium concentration of the thaulla area at average depth (0-30 cm) changed from 50.55 to 175.67 ppm with average K concentration of 96.10 ppm. However, the K concentration was observed to be increasing towards the water spread area of the tank without clear trend (Fig. 3). In addition, the average K concentration of tank water during the study period (12.15 ppm) was lower than the thaulla area. This behavior of K in the thaulla area is different from other elements such as Na, Ca, and Mg. Shen and Stucki (1994) indicate that when the Fe in smectites (a type of clay mineral) is reduced K fixation is increased and likely results in reduced K availability. They also showed that reduction of Fe in illites (a type of clay mineral) results in K release and increases in exchangeable K. Therefore, the clay soils with decreasing trend of Fe concentration toward the water spread area of the tank may affect on this behavior of increase in concentration towards the water spread area of K in the thaulla area. Though higher mud (silt and clay) content towards the water spread area was observed, further studies are needed to identify the clay mineralogy in thaulla area.
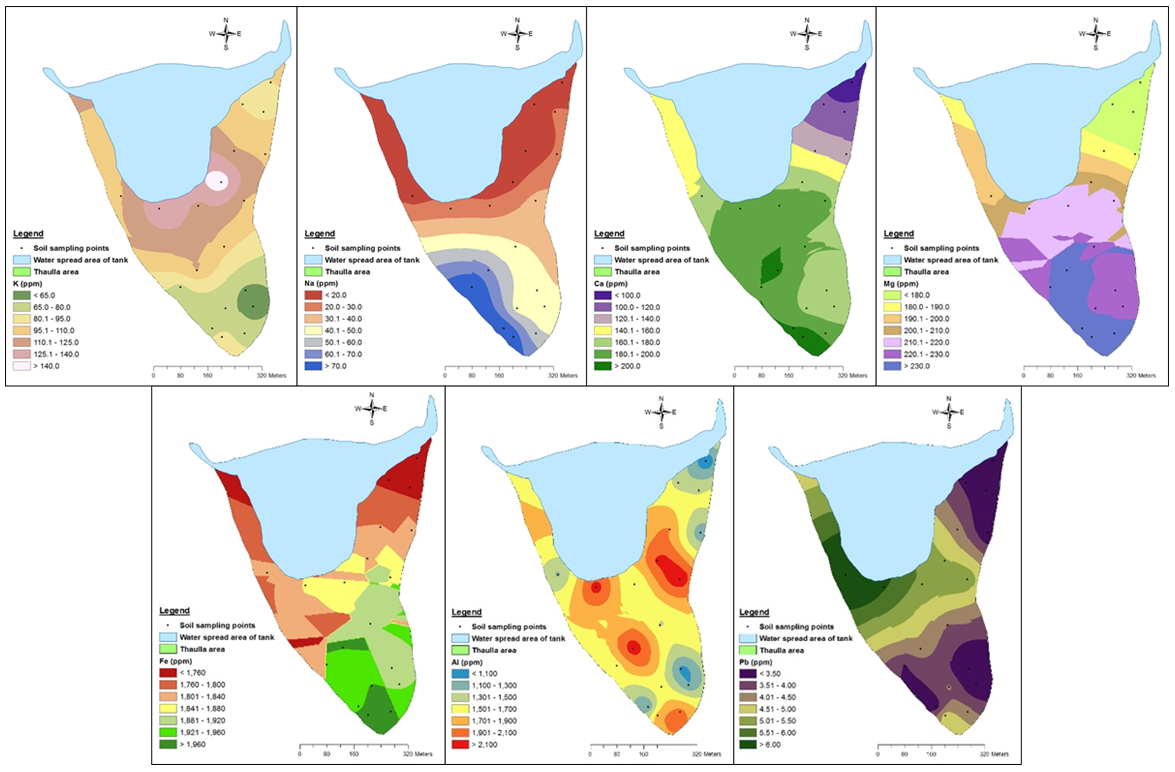 | Figure 3. Variation of average total K, Na, Ca, Mg, Fe, Al and Pb (0–30 cm soil) of thaulla area of Ulankulama tank |
3.6. Variation of Total Sodium (Na), Calcium (Ca) and Magnesium (Mg)
- The total Na, Ca and Mg concentrations of the soil in thaulla area at average depth (0-30 cm) varied from 0 to 108.91 ppm, 61.28 to 370.44 ppm and 109.80 to 349.60 ppm, respectively. Moreover, the average concentrations of Na, Ca and Mg were 37.34, 163.79 and 203.67 ppm, respectively in thaulla area. The concentration of these three elements apparently decreases towards the water spread area of the Ulankulama tank (Fig. 3). This behavior was clear, especially for Na concentration. This reducing trend of basic elements Na, Ca and Mg may be due to absorption of these ions by grasses and also dissolve in water. This uptake process of contaminants from soil or water by plant roots, their translocation and accumulation in aboveground biomass is known as phytoextraction (Kotrba et al., 2009). Also, higher Ca and Mg concentration were observed in the southwestern area of thaulla where there were abundant grasses. It indicates that the thaulla area plays a significant role in trapping basic cations like Ca, Mg and Na.During the dry period, the sodium concentration of the tank water ranged from 31.81 to 104.99 mg/l and the average sodium concentration in the tank water was 52.80 mg/l. Calcium concentration of the Ulankulama tank water varied from 18.28 to 40.82 mg/l during the dry period. The average calcium concentration was 29.22 mg/l. Magnesium concentration of the tank ranged from 10.47 to 19.11 mg/l with an average of 15.26 mg/l during the dry period.
3.7. Variation in Total Iron (Fe) and Aluminum (Al)
- Total iron and aluminum concentrations of the soils of thaulla area of Ulankulama tank at average depth (0-30 cm) changed from 980.2 to 2669.4 ppm and from 898.6 to 2295.2 ppm, respectively. The average concentrations of Fe and Al in soils were 1819.65 and 1586.75 ppm. There were high concentrations of Fe and Al but there was no clear trend for the spatial variation of Fe and Al concentrations in the thaulla area (Fig. 3). The average Fe and Al concentrations of tank water during the dry spell (1.4 and 0.009 ppm) were very low. The geochemistry of tank bed may also influence the variations in ion concentration. The observed high P, Fe and Al concentration of thaulla area may be due to the formation of various compounds of Fe and Al with P in the soil. Wetland soils are effective sinks for many trace metals through precipitation. Moreover, the solubility of Fe plays a major role in wetland environments (Reddy et al., 2000). Yu et al. (2015) found that spatial distribution of Fe-P and Al-P showed a similar order in constructed wetlands. Other researchers have reported a significant relationship between extractable Fe and Al, and the P retention capacity of wetland soils (Lookman et al., 1995). Observed higher concentrations of P, Al, and Fe suggest that thaulla area act approximately as a wetland. Gambrell (1994) indicated that significant amounts of iron and aluminum oxides present in highly weathered and highly oxidized wetland soils are effective in adsorbing most trace metal cations. Therefore, thaulla area can have the ability to adsorb some other trace metals other than Ca, Mg and Na as influenced by higher concentration of Fe and Al.
3.8. Total Arsenic (As), Cadmium (Cd) and Lead (Pb) in thaulla Soil and Water in the Tank
- Arsenic, cadmium, and lead concentrations of Ulankulama tank water during the study period were not in the detection range of the ICP-OES. Wijesundara et al. (2013) found that Cd2+ concentration in Mahakanumulla tank in Sri Lanka, which is closer to Ulankulama tank varied from 0.18 to 0.31 mg/l during a year. Cd2+ concentration elevated in the months of April and May in Yala season while relatively lower values were recorded for the Maha season (Wijesundara et al., 2013). With the chemical fertilizers such as triple superphosphate significant amount of Cd2+ could be added to the soil (Mclaughlin et al., 1996). It is also reported that Cd2+ concentration in buffalo and cattle dung varies 0.5 - 7.8 mg/l (Wijewardana and Gunarathna, 2004). During the rainy periods, dissolved heavy metals can reach the thaulla area of the tank with runoff water. Therefore, it can be suggested that grasses and other aquatic plants in the thaulla area of Ulankulama tank trap and absorb these heavy metals. Aquatic plants can accumulate elements through their roots, stems, and leaves (Jackson, 1998). Many plant species, especially aquatic macrophytes and some wetland plants have shown promising ability to uptake arsenic from soil and reduce it as metal chelates using specific high-affinity ligands (like oxygen-donor ligands, sulfur-donor ligands, and nitrogen-donor ligands). Bioaccumulation in stems and leaves through phytovolatilization is possible tolerance mechanisms of plants against arsenic contamination (Mirza et al., 2014).The lead concentration of the soil at thaulla area at average depth (0-30 cm) changed from 2.16 to 7.19 ppm with average Pb concentration of 4.14 ppm of. The concentration of Pb apparently increases towards the water spread area of the tank similar to K variation in the thaulla area of Ulankulama tank (Fig. 3). But the average Pb concentration of tank water during the study period was not in the detection range of the ICP-OES (< 5 µg/l). This special behavior of Pb in the thaulla area is similar to K but different from other elements such as Na, Ca, Mg, Fe and Al. Pb tends to accumulate with clay minerals or get adsorbed onto Fe-oxide particles (Chandrajith et al., 2008). In wetlands, heavy metals are reduced effectively due to sorption processes in wetland systems (Yeh and Wu, 2009). In addition, it is shown that wetland can effectively remove heavy metals in water by acting as a matrix, facilitating interaction between microbes and plant/animal communities and performing functions such as filtration, adsorption, precipitation, ionic exchange, microbiological degradation, and biological uptake (Malaviya and Singh, 2012).Water quality of the tank is affected by the hydro and geological processes of the immediate catchment and also the hydro geochemistry of the tank and its bed. Therefore, the variations of some physicochemical parameters along the thaulla area of Ulankulama tank are discussed in this study with the variation in water quality of the tank. The results of these findings suggest that the thaulla area of a tank acts as a small wetland and it plays a significant role in trapping pollutants. This study convinced that thaulla area plays a very important role in the retention of chemical pollutants. Otherwise that would flow directly into the tank and deteriorate the quality of water. This study helps to identify the role of thaulla area of a tank and claim to protect it for continuing its role for sustainable management of tank water for irrigation and other purposes. Moreover, this study shows the importance of the restoration of thaulla areas of dry zone tanks to keep up the good quality water of tanks in Sri Lanka. Further studies are needed to find out the relationships among nutrient retentions, especially P, Fe, Al, and the behavior of K along the thaulla area, with clay mineralogy, organic carbon and other soil quality parameters in thaulla area. Also, analysis of aquatic plants and grasses are needed to find out their functions in the thaulla area particularly the absorption of chemicals in thaulla area. Furthermore, analysis of water samples of the tank during low, medium and high flood levels and compare the results of variations in soil properties are needed to concrete understanding the behavior of thaulla area. Moreover, it is suggested to analyze the total suspended load in water in different water levels along with the runoff water as one of the main function of thaulla is trapping sediments.
4. Conclusions
- The results of this study showed that the thaulla area of a tank acts approximately as a wetland, especially considering the chemical retention function. Soils in thaulla area play a very important role in chemical retention, which prevents flowing of such chemicals directly into the tank water, deteriorating the water quality. This function is evident by the high accumulation of Fe and Al with a considerable retention of P, Ca and Mg in the soil of thaulla area of the Ulankulama tank. The chemical precipitation function of thaulla was further corroborated by observing considerable higher concentrations of P, Fe, and Al than those of the reference values of the soils (Reddish Brown Earth) in the area. Moreover, this function was confirmed by the very low concentrations of Fe, Al, and P in tank water even during the dry season. Thaulla area is functioning as a sink for P as a wetland thus reducing the overall P concentration to the tank. P might retain by various compounds of Fe and Al in the soil similar way of a wetland. N was the limited nutrient in the thaulla area and it was also identical to the wetlands. EC, N, P, Na, Ca, and Mg concentrations decreased towards the water spread area. In contrast, K and Pb concentrations followed the opposite. The reason for this chemical precipitation may be due to the abundance of aquatic grasses and trees in the thaulla area of Ulankulama tank. These findings reconfirm the importance of ecological functions of thaulla area of tanks for sustainable use of tank water.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML