-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2019; 9(2): 36-40
doi:10.5923/j.re.20190902.02

Tick-Wildlife Associations in Lowland Rainforest, Rivers State, Nigeria
Mekeu Aline Edith Noutcha, Chinasa C. Nwoke, Samuel Nwabufo Okiwelu
Entomology and Pest Management Unit, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Samuel Nwabufo Okiwelu, Entomology and Pest Management Unit, Department of Animal and Environmental Biology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background and Objectives: Ticks and tick-borne diseases are a major constraint to livestock production in sub-Saharan Africa. Wild meat (bushmeat) is meat from any wild terrestrial mammal, bird, reptile or amphibian harvested for subsistence or trade, most often illegally. The benefits of bushmeat are varied. A compelling case has been made that our focus on wildlife should not be restricted to conservation but extended to their health. Many of the studies on tick-wildlife associations on the continent were in South Africa. Studies in Nigeria are very limited. An investigation was conducted to determine the tick diversity, numbers, host species and predilection sites on carcasses brought to a rural bushmeat market in lowland rainforest, Rivers State, Nigeria. Methods: The catchment area of offtakes was approximately 54km2. The study was conducted over a 4-month period, late dry and early rainy seasons, March-June, 2010. Specimens, collected directly with forceps from the terrestrial animals, were identified by standard keys. Results: A total of 671 ticks were collected from ten mammalian species. Six ixodid tick genera (Amblyomma, Cosimiomma, Hyalomma, Aponomma, Boophilus, Rhipicephalus, Haemaphysalis) of the 12 on the continent were recorded. The two argasid tick genera on the continent Ornithodorus and Argas were collected. Ticks were found on the 10 wildlife species. The highest numbers were collected from the Greater Cane rat, Thyronomys swinderianus; Maxwell’s Duiker, Cephalophus maxwelli, and Brush-tailed porcupine, Artherurus africanus, in descending order. The least number was on the Mona monkey, Cercopitheus mona. Although head and trunk were preferred to legs as predilection sites, the differences were not significant. These tick genera contain species important in disease transmission. Interpretation and Conclusion: Results were compared to those obtained in 1990 at the same location. In 1990, most ticks were collected from hyaena, Red river hog, palm civet, but in the present study they were from Greater cane rat, Maxwell’s duiker and brush-tailed porcupine. Possible reasons for these differences are discussed. Populations of hyaena probably declined steeply to very low numbers so that the probability of encounter was extremely low or local extinction occurred over the 2-decade period. Since there was a plethora of disease vectors, significant increases in species richness and numbers, it was therefore likely that diseases might have contributed to the decline in wildlife numbers. The genera/species richness of ticks on wildlife was high compared to that on domestic livestock. This was probably related to their wide-ranging movement.
Keywords: Ticks genera, Wildlife species, Predilection sites, Lowland rainforest, Nigeria
Cite this paper: Mekeu Aline Edith Noutcha, Chinasa C. Nwoke, Samuel Nwabufo Okiwelu, Tick-Wildlife Associations in Lowland Rainforest, Rivers State, Nigeria, Resources and Environment, Vol. 9 No. 2, 2019, pp. 36-40. doi: 10.5923/j.re.20190902.02.
Article Outline
1. Introduction
- Ticks and tick-borne diseases are a major constraint to livestock production in sub-Saharan Africa, including Nigeria. Ticks are haematophagous; their veterinary importance is related to the blood-feeding. Their effects are direct and indirect. In cattle, high tick load can lead to weight loss, reduction in milk yield, anaemia, stress, toxicosis, etc [1]. Some tick species act as vectors of a variety of pathogens, causative organisms of major tick-borne diseases in tropical Africa [1]. Consequently, over the past decades, there had been numerous studies on ticks and tick-borne diseases in Nigerian livestock [2-10].In Nigeria, these genera of hard (Amblyomma, Hyalomma, Biophilus, etc) and soft (Ornithodorus, Argas, etc) ticks were collected across eco-vegetational zones: Plateau highlands [7], Sahel [8] and savanna woodland [11]. The dominance of Amblyoma genus was also confirmed by Musa et al. [8], in the Sahel and by Dipeolu and Adeyefa [11] in the savanna woodland. However, in the Plateau highlands, the dominant genus was Rhipicephalus. Studies on tick-wildlife associations or tick-borne diseases of wildlife in Nigeria are very limited [12-14].Wild meat (bushmeat) is meat from any wild terrestrial mammal, bird, reptile or amphibian harvested for subsistence or trade, most often illegally. Bushmeat contributes to the food security and livelihoods of millions of individuals in developing countries [15]. The benefits of bushmeat are varied. Bushmeat provides a key source of protein in areas where domestic livestock are scarce and expensive [16]. It is estimated that it contributes 80-90% of the animal protein consumed in some rural areas in West and Central Africa [17]. It provides an important source of income in rural areas where other income-generating activities are non-existent [18]; it is often favoured for consumption because it is traditional or enhances social prestige [19] or preferred for its taste [20]. Cawthorn and Hoffman [21] made a compelling argument that while we continue with attempts to reverse the decline in wildlife populations as a result of over-exploitation; we must realize that bushmeat management will ultimately depend on understanding and working with people. It is therefore imperative that our focus is not restricted to conservation but extended to the health of wildlife.Many of the recent studies on tick-wildlife associations on the continent were from South Africa [22-26]. Reports on tick-wildlife associations from Nigeria are very limited and not current. An investigation was therefore conducted to determine the diversity, numbers and predilection sites on wildlife carcasses brought to a rural bushmeat market in lowland rainforest, Rivers State, Nigeria.
2. Materials and Methods
2.1. Study Area
- The bushmeat market is located at Omagwa (4°58′N, 6°55′E) along a major interstate highway, 23m above sea level in the Ikwerre Local Government Area, Rivers State, Nigeria. The catchment area for offtakes is approximately 54km2 in lowland rainforest.
2.2. Methods
- The study was conducted over a 4-month period, late dry to early rainy season, March-June, 2010. Collections were made from the carcasses of ten mammalian species. These were: Thyronomys swinderianus (Greater Cane Rat), Cephalophus maxwelli (Maxwell’s duiker), Artherurus africanus (Brush-tailed porcupine), Critecetomys emini (Emin’s Giant Rat), Tragelophus spekei (Situanga), Cercopithecus mona (Mona Money). Viverra civetta (Two-spotted palm civet), Genetta poensis (Forest Genet), Potamochoerus porcus (Red River Hog), Nandinia binatata (African Civet Cat). Each carcass was examined immediately on arrival. Ectoparasites were removed with forceps from the head, trunk (dorsal and ventral) and legs. Collected specimens were placed in labeled containers and preserved in 70% Ethanol. Specimens were identified with standard keys26 and type specimens in the Arthropod Museum of the Department of Animal and Environmental Biology, University of Port Harcourt, Nigeria.
3. Results
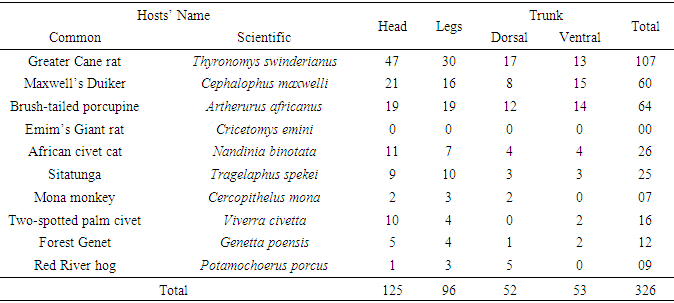
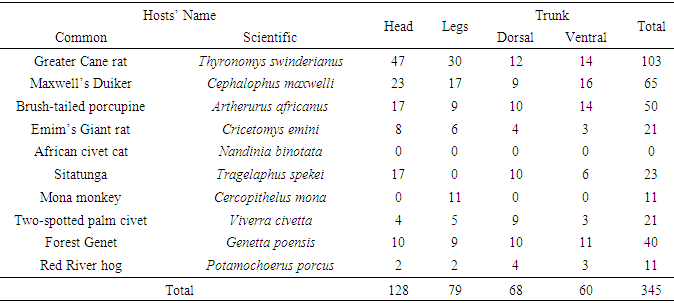
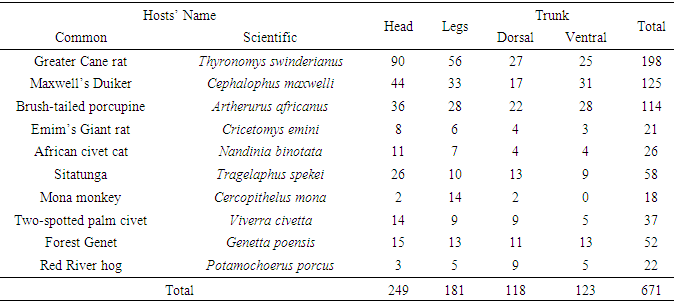
- A total of 671 ticks were collected. Genera recorded were: Amblyomma, Hyalomma, Aponomma, Boophilus, Rhipicephalus, Haemaphysalis (Hard ticks) and Ornithodorus, Argas (Soft ticks). The dominant genera were Amblyomma (39%) and Aponomma (35.66%). The numbers of ticks in the various genera differed significantly (F=1.83, df=89, P<0.05). There was also a significant difference in the number of ticks collected between the late dry and early rainy season (F=0.91; df=1; p<0.05).The highest numbers of ticks were collected from the Greater Cane Rat (Thyronomys swinderianus), Maxwell’s Duiker (Cephalophus maxwelli) and the Brush-tailed porcupine (Artherurus africanus) in descending order. The least number was on Mona monkey. The number of ticks on the hosts’ body (Head, Trunk, Legs) varied with the seasons: 94-123 ticks in the late dry season (Table 1) and 77-126 ticks in the early rainy season (Table 2). The ticks also exhibited seasonal variations in host preference. The highest number of ticks collected during the late dry and early rainy seasons was on the Greater Cane Rat. In the late dry season, the lowest number of ticks collected was on Mona monkey and Emin’s Giant rat while in the early rainy season it was on Mona monkey and African Civet Cat (Tables 1 and 2). Although there was a preference for the head and trunk, the differences in numbers among the attachment sites were not significant (F=0.1, df=19, p>0.05). Furthermore there was no significant difference in numbers of ticks on dorsal or ventral parts of the trunk (F=0.87, df=28, p>0.05) (Table 3).
|
|
|
4. Discussion
- Seven of the twelve genera (Amblyomma, Aponomma, Boophilus, Cosimiomma, Dermacentor, Haemaphysalis, Hyalomma, Ixodes, Margaropus, Nosomma, Rhipicntor, Rhipicephalus) of Ixodid (hard) ticks on the continent and two genera (Argas, Ornithodorus) of Argasid (soft) ticks were recorded [27]. This was a significant increase in diversity from the 1989/1990 study, when only two genera (Boophilus, Hyalomma) were identified [14]. It is apparent that with habitat modification (agriculture, road construction, logging, etc.) and increase in offtake numbers by hunters, wildlife feeding and reproductive ranges were expanded and led to attachment by various genera of questing ticks. The probability of exposure to tick-borne diseases was demonstrated by Sitatunga being susceptible to Cowdria ruminatum. Higher numbers were collected in the rainy season because of more favourable environmental conditions for tick and host development band survival. Similar observations were made in studies on domestic livestock [5,6,10].The consistent highest numbers of ticks on the Greater Cane Rat and to a lesser extent on Maxwell’s duiker was probably related to the numbers of carcasses examined. Studies in the catchment area showed that the Cane rat, Maxwell’s duiker and Brush-tailed porcupine were the dominant species displayed at the Omagwa market [28,29] across seasons. Similarly, the low numbers on the Emin’s Giant rat, Mona monkey and the Red River hog (Potamochoerus porcus) were probably related to the low numbers of these species displayed at the market [28,29]. Ticks quest on blades of grass, waiting to attach to hosts. Consequently, grass-dwelling species, such as the Greater Cane Rat and Brushed-tailed porcupine, are more prone to attachment. This might explain the consistently high numbers of ticks on these species. The tree-dwelling behavior of the Mona monkey was also a contributory factor to the number of ticks. High numbers of ticks were recorded on head and trunk. The head contains the mouth and since these species are mainly herbivores, the head is usually fully covered by grasses, which harbor questing ticks that usually attach. The large surface area of the trunk (ventral and dorsal) increases the attachnment surface area for ticks. These might explain the high numbers of ticks on the head and trunk in the present study.The current pattern of occurrence was at variance with the observations in 1990, when the highest numbers of ticks were caught from Hyaena, Red River hog and Palm Civet; they had the highest frequencies of occurrence at that time. Their numbers are currently very low; in fact, there had been no record of hyaena since 2005. Ticks have also been collected from the scrub hare, Lepus saxilis [22], Nyalas, Tragelephus angasi [23] and eastern rock sengi, Elephantulus myurus [26] in South Africa.The hyaena population must have declined so steeply that the probability of their capture was lpw or they had become locally extinct. Based on the occurrence of a full complement of genera containing varied disease-transmitting-species, it is likely that tick-borne disease may be an important mortally factor in wildlife survival. Strikingly, higher genera/species diversity on ticks than on domestic livestock in Nigeria.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML