-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2018; 8(5): 232-240
doi:10.5923/j.re.20180805.03

Pathogenic Parasites in Raw and Treated Wastewater in Africa: A Review
Abdallah Zacharia 1, Anne H. Outwater 2, Billy Ngasala 1, Rob Van Deun 3
1Department of Parasitology and Medical Entomology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
2Department of Community Health Nursing, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
3Unit Life Sciences and Chemistry, Thomas More University of Applied Sciences, Belgium
Correspondence to: Abdallah Zacharia , Department of Parasitology and Medical Entomology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Wastewater is reused for irrigation in agriculture in many African cities. However, the use of partially/untreated wastewater may result in the transmission of infectious organisms such as parasites. This article reviews the prevalence and concentrations of parasites in raw and treated wastewater in African countries and the efficiency of the wastewater treatment systems used. This will highlight the burden of parasitic infections in African communities and suitability of reusing wastewater in these communities. The following databases: PUBMED, HINARI and Google Scholar were searched for any article presenting information on the occurrence and concentration of parasites in wastewater in any African country. No restrictions were set on date of publication, study design or language. Thirty publications were identified. These publications presented works related to parasites in wastewater in 12 African countries. A total of 23 parasite species were identified throughout the 5 African regions. Eggs of Ascaris lumbricoides predominated followed by Hymenolepis species, Trichiuris trichiura, Hookworm, Taenia species, Enterobius species, Toxocara species and Schistosoma species. Cysts of Giardia species, Entamoeba histolytica and Entamoeba coli were the most commonly identified protozoa. Waste stabilization ponds and activated sludge systems are the common wastewater treatment systems used in Africa with the former being more efficient for parasites removal than the later. The review results show that wastewater in Africa contains a variety of pathogenic parasites with protozoa predominating helminth and putting public health at risk. Despite the fact that wastewater treatment systems removed helminths, some of them did not meet the WHO standard. Moreover, these systems do not clear protozoa and there is no standard concentration established for safe reuse of wastewater. Therefore, there is a need for improving treatment systems design and management. Moreover, standards for protozoa should be set.
Keywords: Wastewater, Africa, Parasites, Helminth, Protozoa
Cite this paper: Abdallah Zacharia , Anne H. Outwater , Billy Ngasala , Rob Van Deun , Pathogenic Parasites in Raw and Treated Wastewater in Africa: A Review, Resources and Environment, Vol. 8 No. 5, 2018, pp. 232-240. doi: 10.5923/j.re.20180805.03.
Article Outline
1. Introduction
- Wastewater reuse is a common practice in Africa. About 10% of the population in African cities engages in urban agriculture and uses wastewater as a source of irrigation water [1]. In Africa, wastewater irrigation ranges from the use of polluted surface water to raw wastewater to the piped distribution of secondary or tertiary treated wastewater [2]. Apart from being a reliable quantitative source, wastewater is usually found close to urban markets and contains useful nutrients which improve soil fertility [2]. However, the use of both treated and untreated wastewater poses risks of transmission of diseases to farmers and the community [3], [4]. Parasites are one of the most frequently identified pathogenic organisms in wastewater [5]. Parasites’ infective stages like eggs, cysts and oocysts are also environmentally resistant stages which can help them resist different types of wastewater treatment processes and facilitate their survival [6, 7]. This is mainly due to the presence of strong outer layer(s) which protect them from various physical and chemical destructions. Pathogenic parasites can persist and remain viable in wastewater for a long period of time compared to bacteria and viruses. Protozoa parasites can survive in wastewater up to one month while helminths can survive up to twelve months [8]. This period is sufficient for them to be transported to the farmland where further contamination to humans and materials consumed by humans may occur. Moreover, pathogenic parasites require a very low infective dose to cause an infection to the new host. As few as one helminth egg or protozoa (oo)cyst is enough to act as an etiological agent for a particular parasitic disease [9, 10]. World Health Organization (WHO) advises that wastewater that will be used to irrigate edible crops, sports fields, public parks, cereal crops, industrial crops, fodder crops, pasture and trees, should be restricted to a helminth level of ≤ 1 egg per litre. The main objective of this article is to review the prevalence and concentrations of pathogenic parasites in raw and treated wastewater in different parts of Africa and the efficiency of the available wastewater treatment systems in removing these parasites.
2. Methodology
- A thorough literature search was conducted in the following databases: PUBMED, HINARI and Google Scholar. The keywords used were: “wastewater”, “sewage”, “parasite”, “helminth”, “protozoa”, “occurrence” and “Africa” to create search query by using “AND” and “OR” operators. We restricted our search to original research articles presenting research work conducted in the African continent. No restrictions were made in the year of publication, language or study design. All articles presenting information on the prevalence (occurrence) and/or concentration of at least 1 parasite species in domestic wastewater or water bodies contaminated with domestic wastewater were selected. A further hand search within the bibliography of all selected articles was conducted for the identification of any important additional articles.Tables were formulated to aggregate information on the name of the first author and year of publication, the country where a study was done, the volume of wastewater sample used, type and source of wastewater and diagnostic method(s) used. Moreover, we gathered information on the wastewater parasites occurrence and the mean concentration. The articles which did not present these information, data were re-analysed to obtain the required information. The percentage parasites reduction was considered as the parasites removal efficiencies. It was calculated by taking the difference in parasites means concentrations between the inlet and outlet of a particular treatment system divided by the mean parasites concentration of the inlet times 100. Statistical software PAST 3.20 was used to compare parasite concentrations between different locations and types of wastewater treatment systems, and parasites removal efficiency among different types of wastewater treatment systems.
3. Results
3.1. General Findings
- Based on our search query we discovered 194, 1973 and 4500 total number of search hits in PUBMED, HINARI and Google Scholar databases respectively. Of these we were able to access 194, 500 and 1000 publications in PUBMED, HINARI and Google Scholar respectively with either title and/or abstract. From this search we found 25 peer-reviewed research articles that met our eligibility criteria. From the bibliography of some of the selected 25 articles, 5 more important ones were identified and included, making a total of 30 articles.These 30 articles present original research works conducted in 12 African countries. The following are the 12 African countries with the number of articles in brackets: Tunisia (5), Morocco (5) and Egypt (3) in Northern Africa; Burkina Faso (4), Ghana (2), Ivory Coast (1) and Nigeria (2) in Western Africa; Cameroon (2) in Central Africa; Uganda (1) and Kenya (3) in Eastern Africa; and Lesotho and South Africa (1) and South Africa (1) in Southern Africa.
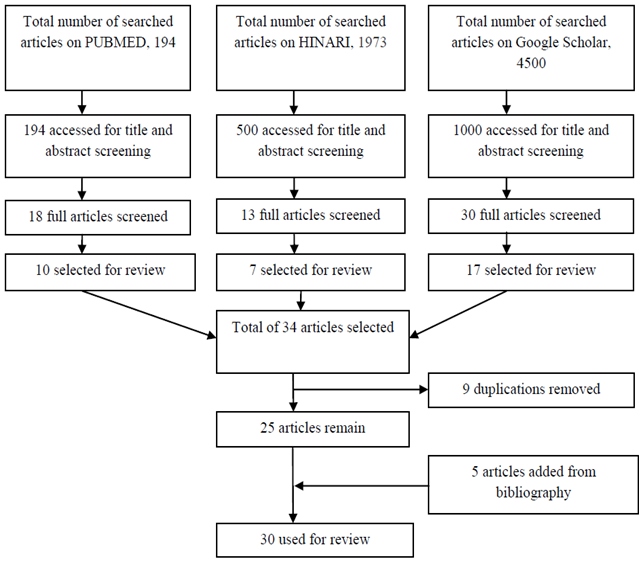 | Figure 1. Illustrates the steps followed in the articles selection |
|
3.2. Parasites Identification
- A total of 23 parasitic species were identified throughout the studied areas. These species consist of 17 helminths and 6 protozoa. Of 17 helminth species: 9 were nematodes, 5 were cestodes and 3 were trematodes. Regardless of the number of articles identified in each region, more species were identified in northern Africa (18: 13 helminths and 5 protozoa) followed by western Africa (17: 13 helminths and 4 protozoa). Studies conducted in central Africa have identified a total of 6 types of species whereby 5 were helminths and 1 was protozoa. Studies in eastern and southern Africa each have identified 7 types of species of which 5 were helminths and 2 were protozoa. Ascaris lumbricoides has been identified in 26 studies aimed at assessing helminths or Ascaris species in wastewater. It is followed by Hymenolepis species (19 studies), Trichiuris trichiura (16 studies), Hookworm (15 studies), Taenia species (15 studies), Enterobius species (11 studies), Toxocara species (7 studies) and Schistosoma species (5 studies). Giardia species, Entamoeba histolytica and Entamoeba coli are the most frequently identified protozoan detected in 15, 11 and 11 studies respectively. The remaining parasites were diagnosed in less than 5 studies only. Geographically, Ascaris lumbricoides, Trichiuris trichiura and Taenia species were the parasites that have been identified in at least 1 study conducted in each of the 5 African regions. Parasites which were identified in at least 1 study conducted in 4 regions include Hookworm species, Cryptosporidium species and Giardia species. Enterobius species and Hymenolepis species were identified in only 3 regions. Entamoeba histolytica, Entamoeba coli and Fasciola species were found in only 2 parts of Africa. The remaining 10 species have been detected in only 1 region of Africa.The study conducted by Sabbahi [16] in Tunisia has identified 11 parasite species which is the highest number of parasites species detected in a single study compared to any of the other studies. It is followed by studies conducted by Salama [12] in Morocco and Okojokwu [30] in Nigeria whereby 10 parasites species were identified in each study. Others is as follows: 2, 1, 6, 6, 5, 1, 2, and 4 studies each detected 9, 8, 7, 6, 5, 4, 2 and 1 parasitic species respectively.
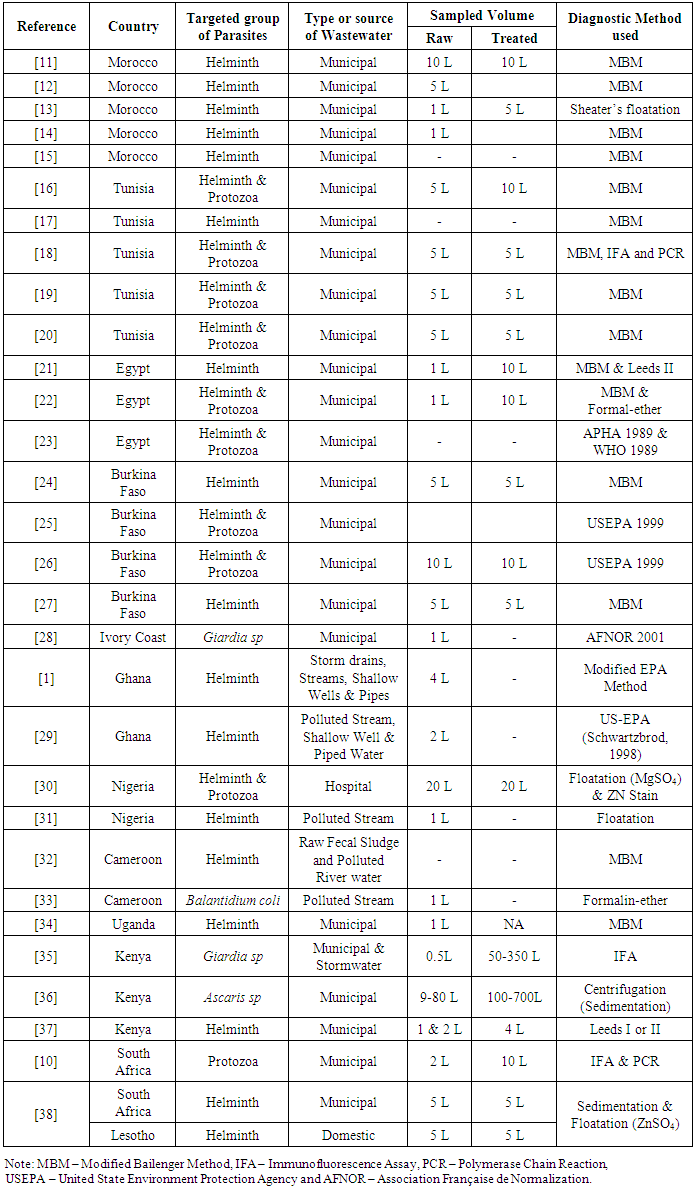
3.3. Parasites Concentrations in Raw and Treated Wastewater
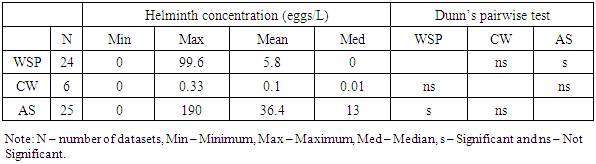
- Table 2 presents the summary statistics of data from 22 articles presented information on mean helminth concentrations in raw wastewater of various sites. No information was found for the helminth concentration in raw wastewater in central Africa region. A Kruskal Wallis test provided strong evidence of difference in helminth concentrations between the 4 remaining African regions (H (2) = 12.62, p = 0.006). A Dunn’s pairwise test was carried out for the four pairs of groups. There were strong evidence of the difference in helminth concentrations in raw wastewater between northern and western Africa (p=0.022), and northern and eastern Africa regions (p=0.002).
|
|
|
|
|
|
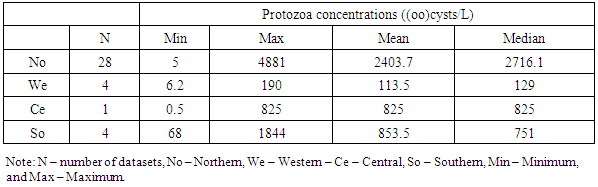
3.4. Parasites Removal Efficiencies by Wastewater Treatment Systems in Africa
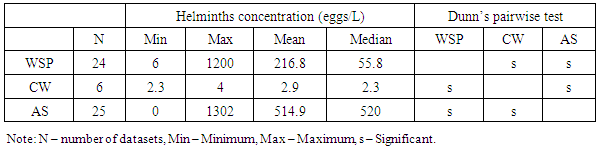
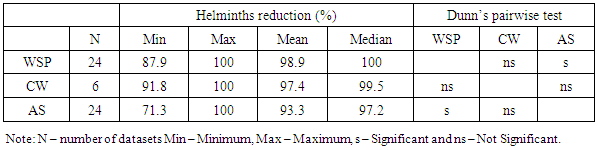
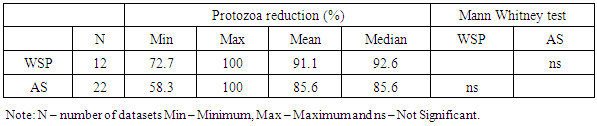
- Wastewater treatment for parasites removal ranges from the use of mechanical processes (conventional) to natural treatment systems. Most of wastewater treatment systems only treat parasites’ eggs, cysts and oocysts by separating them from suspension in wastewater into sediments and sludge and not their destruction or inactivation. The postulated processes associated with parasites removal in most wastewater treatment systems include sedimentation, filtration, predation, adsorption and absorption. Waste stabilization ponds, activated sludge and constructed wetlands were the types of wastewater treatment systems that were observed to be commonly used in Africa. Waste stabilization ponds are man-made shallow basins comprising a single or series of anaerobic, facultative or maturation ponds. Anaerobic ponds are used as a pre-treatment. They receive high organic loads. These high organic loads produce anaerobic conditions throughout the pond. Facultative ponds are used as a secondary stage. Remaining biodegradable organic matter is removed through the coordinated activity of algae and heterotrophic bacteria. Maturation ponds are used as tertiary treatment. The main function is the removal of pathogens and nitrogen. Constructed wetlands are man-made wetlands built to remove various types of pollutants present in wastewater that flows through these systems. They are constructed to recreate the structure and function of natural wetlands. They possess a rich microbial community to effect the biochemical transformation of pollutants, they are biologically productive and most important, they are self-sustaining. An activated sludge treatment system is a technological, mechanical wastewater treatment process for treating different types of wastewater using aeration blowers and a biological floc composed of bacteria and protozoa. There is variation in parasites removal efficiencies between the three types of wastewater treatment systems. A Kruskal Wallis test for equal medians provided strong evidence (H (2) = 6.02, p = 0.002) of difference in percentage helminth reduction between the 3 groups of treatment systems. Dunn’s pairwise test shows that, there was strong evidence of the difference in helminth reduction between WSP and AS (p=0.005). There was no evidence of difference between the other pairs.
|
|
4. Discussion
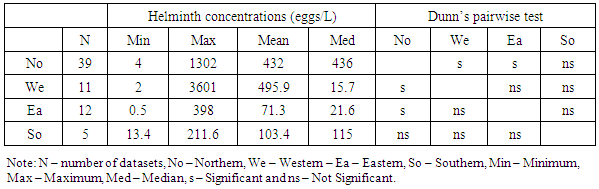
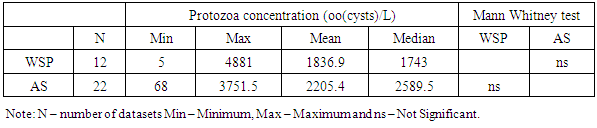
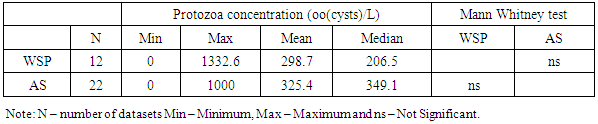
- Raw wastewater in Africa contains many species of pathogenic parasites from human and animal contaminations. Eggs of Ascaris lumbricoides predominated followed by Hymenolepis species, Trichiuris trichiura, Hookworm, Taenia species, Enterobius species, Toxocara species and Schistosoma species. Cysts of Giardia species, Entamoeba histolytica and Entamoeba coli are the most commonly identified protozoa. To some degree it agrees with Stott [21] who stated that eggs of Ascaris are usually predominating in raw wastewaters followed by Trichuris species, hookworm, Hymenolepis species, Taenia species, Toxocara species and Enterobius species for the helminths while Giardia species and Cryptosporidium species lead for the protozoa parasites. The occurrence and concentration of these parasites have shown to vary between different parts of Africa. The substantial variation of parasites concentration is attributable to the difference in concentration of parasitites excreted by sewered communities and their animals to the wastewater and/or difference in percentage volume of non domestic wastewater such as industrial and stormwater channeled to the sewage systems.In this review we have observed that intestinal parasites in wastewater were distributed throughout all regions of African continent with higher concentrations reported in northern Africa. This region appears to have higher production of wastewater and more direct use of raw and treated wastewater than other parts of Africa [39]. According to Jimenez et al., high consumption of wastewater due to high water demand for irrigation leads to the proliferation of parasitic diseases [40].The mean parasites concentrations in raw wastewater of Africa exceed those reported from other parts of the world. For example the mean helminth concentration obtained in studies conducted in Africa range from 0.5 to 3601 eggs per litre (Table 2) which supports Jimenez and colleagues [40] reported that helminth concentration in wastewater in most low and middle income countries range from 70-3000 eggs per litre and in high income nations range from 1-9 eggs per litre. However, there are place that have reported higher mean concentration of helminth parasites compared to that reported in Africa. For example, from a shanty district in Pedregal, Brazil a mean concentration of 16,774 eggs per litre of helminth parasites were detected in raw domestic wastewater [41].Protozoa parasites were observed to occur at higher concentrations than helminths in almost all African regions (Tables 2 and 3). The predominance of protozoa parasites may be due to their higher prevalence [19]. This is confirmed by local epidemiological data presented in specific countries. For example epidemiological data collected from studies done on diarrheic people and food handlers in Tunisia, revealed larger number of protozoa than helminth [16]. Indeed up to this moment the control of these parasites has been neglected. Measures for the control of wastewater helminth parasites transmission were put in action since the late 1980s when the WHO developed guidelines for water and sludge reuse in agriculture [40]. Moreover, other control measures that are not related to wastewater use have been put by WHO to control intestinal helminths in many African countries, including mass drug administration to the most vulnerable groups [42]. Lack of control measures may be the reasons of ubiquitous presence of protozoa and the higher prevalence in wastewater in Africa.The parasites removal efficiencies and mean concentrations for treated wastewater in African treatment plants also vary between the types of treatment systems. According to WHO guidelines, treated wastewater should contain ≤ 1 egg per litre of helminth to be suitable for use in restricted and unrestricted agriculture [43]. For protozoa parasites, a reduction of 6-7 log units for unrestricted agriculture and 2-3 log units for restricted agriculture is required [43]. The summarized results of parasites mean concentration for treated wastewater in Tables 5 and 7 show that all types of wastewater treatment systems in Africa reduced parasites concentrations to a certain extent. However, only constructed wetlands (CW) systems consistently met WHO helminth standards. Moreover, removal of protozoa in other types of systems also showed unsatisfactory results of ≤log 1 (≤90%) reduction (Table 9).We also observed that helminth and protozoa concentrations in effluents of the treatment systems varied with the initial concentration of the parasites in their influents. The concentration in effluent is high in most systems with high influent concentration for both protozoa and helminths, and low in most systems with low initial concentrations. A similar observation was reported in Brazil whereby wastewater treatment by using a UASB reactor produced high helminth concentration (1740 eggs/l) due to the large concentration in the raw wastewater (16774 eggs/l), despite its high rate of parasites removal [41]. In this review, this is seen well in Tables 4, 5, 6 and 7 which summarize the results of mean helminth and protozoa concentrations in the treatment systems.The type of treatment system also showed an effect on the concentration of parasites in their effluent. Effluents of WSP systems were observed to contain lower parasites concentrations compared to those of AS and CW systems. However, for the helminths the difference was found to be significant only between WSP and AS systems, while for the protozoa the difference was found to be not significant. Generally, in treated wastewater, the concentration of protozoa was significantly higher than helminths. The predominance of protozoa parasites may be due to their high concentration in inlets wastewater (Tables 4 and 6), high resistance to treatment systems, and/or their small size [20].As in the case of parasites reduction, there were differences in removal efficiencies between various wastewater treatment plants in Africa. WSP systems from studied areas achieved a mean helminth percentage reduction of 98.9%. Studies conducted in countries outside Africa reported almost similar percentages of helminth reduction. Verbyla recorded 97% overall average reduction in Bolivia [44]; 100% removal was reported by Dixo and Mirzaei in Brazil and Iran respectively [41, 45]. Only a few studies explored protozoa removal by WSP systems in Africa but showed that the systems managed to remove this type of parasites by 90.3%. Activated sludge (AS) systems, one of the two commonest types of wastewater treatment systems used in Africa, were observed to remove helminths at the mean of 97.4% and protozoa at the mean of 85.6%. AS percentage removal of helminths is significantly lower than that achieved by other types of treatment systems in the continent while the difference for protozoa reduction is not significant Moreover, the performance of AS systems in Africa is higher than the expected average removal of 0.65 log10 (78%) of helminths and lower than the expected reduction of 1.3 log10 (95%) for protozoa [46].A CW system is a natural wastewater treatment system which has been assessed in few African countries. They have showed high parasites removal specifically helminth eggs. CW systems are commonly used as secondary treatment systems and receive wastewater after passing through pretreatment processes. The fact that they have received helminths at low concentrations may be due to the effect of pretreatment. This type of treatment system removes helminth parasites at higher percentage than AS systems and almost similar to WSP.Despite the fact that some wastewater treatment systems in Africa achieved greater than 90% reduction of both protozoa and helminthic parasites, some did not meet the set standard for parasites removal (≤1 egg/l of helminth) and therefore producing effluents that may be unsuitable for use in unrestricted and/or restricted irrigation.
5. Conclusions and Recommendations
- In Africa, wastewater is a reliable source of water which is often used for irrigation purpose, especially in those areas with limited availability of fresh water. However, proper treatment is inevitable to protect the public from at the risks of acquiring wastewater transmissible pathogens including parasitic helminths and protozoa, which are ubiquitous throughout the African continent. To achieve this, it is necessary to treat all wastewater by well-designed treatment systems that can remove highly resistant pathogenic organisms such as helminths and protozoa. Moreover, to ensure low risk of parasites transmission by wastewater, it is recommended that in addition to indicator bacteria, protozoa cysts be used as indicators of water quality. Given the high public health risk they present to the general population because of their high concentrations in wastewater. Standards for protozoa wastewater effluent concentrations should be set.
ACKNOWLEDGEMENTS
- This study was supported by the South Initiatives project “Pathogen removal from wastewater using sustainable treatment wetlands”, funded by VLIRUOS, Brussels, Belgium.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML