-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2018; 8(2): 68-72
doi:10.5923/j.re.20180802.07

Bioassay of Residual Fluridone Following Prolonged Wet and Dry Cycles in Coastal Plain Soil of Niger Delta
Mohammed K. Hamadina 1, Elsie I. Hamadina 2
1Soil Science Department, Faculty of Agriculture, University of Abuja, FCT, Nigeria
2Crop and Soil Science Department, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria
Correspondence to: Mohammed K. Hamadina , Soil Science Department, Faculty of Agriculture, University of Abuja, FCT, Nigeria.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Decades ago, Fluridone was formulated to control aquatic weeds. Recently, Fluridone is used in manipulating plant physiological processes, and also as terrestrial herbicides. Fluridone may enter the soil through different pathways; but its persistence in soils is not clearly understood, especially following a prolonged dry period. Bioassays using responsive plants species are useful in assessing carryover effects of herbicides. Fluridone (three applications of 100 ml of 30 µmol solution each) and No Fluridone control were subjected to 30 days of wet and dry cycles, followed by 77 days of dry periods, and thereafter planted with maize (variety Oba Super 6), i.e., after rewetting the soils. Height and chlorophyll measurements were done on the maize seedlings at 5 DAP and 10 DAP. The morphology of the seedlings was observed throughout the duration of the bioassay study. The shoots of the maize seedlings were harvested at 10 DAP, roots sampled by washing off the soil, and both shoot and roots were oven dried to constant weight. Seedling heights did not differ, but seedlings growth rate from 5th to 10th DAP declined drastically as compared to the period before 5 DAP. Leaf chlorophyll at 10 DAP was significantly reduced in seedlings growing in SA treatments. The leaves of seedlings growing in SA treatment began to whiten by 6th DAP and the bleaching effect progressed up to 10 DAP, apparently manifesting the reduced chlorophyll content. The shoot dry matter contents, root dry weight and shoot:root of the maize seedlings varied significantly (P<0.05) with treatment. This study showed that despite prolonged soil drying preceded by wetting and drying cycles, Fluridone persisted in soil with carryover effects on maize. Therefore, Fluridone is not recommended for soil application in the humid tropics due to its potential to persist in the soils.
Keywords: Residual effects, Fluridone, Humid tropical Soils, Maize seedling
Cite this paper: Mohammed K. Hamadina , Elsie I. Hamadina , Bioassay of Residual Fluridone Following Prolonged Wet and Dry Cycles in Coastal Plain Soil of Niger Delta, Resources and Environment, Vol. 8 No. 2, 2018, pp. 68-72. doi: 10.5923/j.re.20180802.07.
Article Outline
1. Introduction
- Fluridone is an aquatic herbicide containing 41.7% 1-methyl-3-phenyl-5-[3-(trifluoromethyl)phenyl]pyridine-4-one (C19H14F3NO) with no reports of toxicity to the aquatic systems when used according recommendations [1]. More than four decades ago, when Fluridone was being formulated, it was found to be efficacious (but not cost-effective) in controlling weeds in cotton fields [2, 3]. In addition, Fluridone was found to have adverse carryover effects on several crops including maize, peanuts, rice, sorghum, and tomato [4, 2]. Fluridone was eventually adapted for the control and management of aquatic weeds [1].Fluridone works by inhibiting the enzyme phytoene desaturase, which plays critical roles in the biosynthesis of carotenoids [5-7], eventually suppressing abscisic acid production in the affected plants. Interestingly, this ability to alter the biochemical synthesis of abscisic acid has turned the attention of scientists to the potentials of fluridone for use manipulating physiological processes in both plants and animals. Fluridone has shown promising results in breaking dormancy in inherently dormant plants [8], [9]. Also, Fluridone is being tested for potential use as analgesic drug on mammals [10].In recent times also Fluridone is increasingly being employed for the control of herbicide resistant weeds on arable lands [2, 3], applied at pre-planting, pre-emergence or post-emergence. Whichever way Fluridone is applied to crop fields, it eventually finds its way into the soil through, direct or indirect pathways [11, 12]. These pathways may include application in irrigation water, direct soil application as pre-planting or pre-emergence herbicide. Indirectly Fluridone or its derivatives enter the soil through exudates from plants that received Fluridone via foliar sprays, or as aerosols falling unto the soils from foliar sprays [12]. Fluridone, being a weak base, gets adsorbed to organic matter and clay minerals in soil, and this phenomenon is regulated by the pH, temperature, organic matter, and clay and moisture contents of the soil [13]. However, the persistence of Fluridone in soils is still not clearly defined, although reports of studies on carryover effects show adverse effects on some arable crops planted immediately after the harvest of the target crop.Toxic effects of terrestrial herbicide are usually determined using bioassays for germination/emergence and vigour of dicotyledonous or monocotyledonous seedlings [1]. Leaf chlorophyll is an indicator of crop health that can be employed to diagnose nitrogen deficiency [14], hence, can also be used to detect the effect of effects of Fluridone on fast growing plants such as maize [12]. Using hand-held devices, chlorophyll in leaves can be measured rapidly and non-destructively at minimal costs. In a recent study, [12] reported that planting maize within one month of direct soil application of Fluridone had adverse effects on maize seedlings, including leaf chlorophyll contents at three weeks after planting, due to chlorosis of the leaves. However, little is known about the effect of prolonged soil drying, such as the case during the dry season, on the persistence of Fluridone in soils. Prolonged drying of soil mimics the dry season period when little no rainfall is experienced, which usually follows a cropping season in rain fed farming systems. In such systems, planting resumes at the start of the next rains and usually the first crop is the fast-growing cereal staples such as maize. This study was conducted to determine the residual effects of soil-applied Fluridone following a prolong drying of the soil.
2. Materials and Method
2.1. Experimental Site and Test Soil
- The soil used in this experiment has been described by [12]. The soil used in the experiment is loamy and slightly acidic (pH 5.6), with moderate soil organic matter content. In summary the soil, which was obtained from a bush regrowth, was used in an experiment involving soil-addition of Fluridone at 30 µmol via direct watering or indirectly through plant foliar sprays. At the end of the experiment, the soil was stored under ambient conditions for a month and then used for the experiment of [12] when maize was grown in plastic pots containing 250 g soil for 21 days in a screen-house in October 2017. At the end of the experiment, the soil was subjected to wet and dry cycle through November 2017 and then left to dry in the screen-house until 15th February 2018 when it was rewetted and planted to maize. This means that the soil was incubated in the screen-house for a total 107 days comprising of 30 days of wet and dry, followed by 77 days of continuously dry periods.
2.2. Experimental Design and Treatment
- At the start of this experiment, the dried soil from the previous experiment was wetted (i.e., after 30 days of wetting and drying, followed by 77 days of prolonged drying) to field capacity and sowed with maize (variety Oba Super 6) the next day, on 15 February 2018. The experiment laid in a completely randomised design with three treatments and three replications. The treatments have been described by [12] and they included No Fluridone (NF) Previous Soil-Applied (SA), and Previous Foliar-Applied (FA) Fluridone solution (30 µmol).
2.3. Test Plants
- The Oba Super 6 maize variety was used as test plant. The variety is a single cross maize hybrid produced by Premier Seeds Nig Ltd., and it is adapted to the moist Guinea Savannah, tolerant to low soil nitrogen and yielding as high as 7-8 ton ha-1 [15]. Ten (10 nos.) seeds of Oba-Super 6 were planted per pot to observe germination (seedling emergence) and later pruned to 3 seedlings per pot at 3 DAP.
2.4. Plant Sampling/Data Collection
- Plant height was measured, using a 1.0 mm graduated rule, from the base of the see tip of the longest leaf (by raising the leaves upwards against the rule). Height measurements were carried out at 5 and 11 days after planting (DAP).The chlorophyll contents of the maize seedlings were measured using atLEAF® chlorophyll meter on the same days height measurements were done. The atLEAF® is a simple tool uses a combination of transmission of red (660 nm) and ultra violet (940 nm) light measurements, i.e., at wavelengths where chlorophyll absorbs or does not absorb light respectively [14].The shoots and roots were harvested by neatly cutting the seedlings at the base (soil surface) to obtain the shoots, while and the roots were washed gently to separate the roots from the soil medium. The shoots and the roots were oven-dried at 70°C to constant weight to estimate dry matter contents.
2.5. Statistical Analysis of Data
- The data sets collected in the course of this trial were subjected to statistical analysis of variance (ANOVA) using Genstat® Computer software.
3. Results and Discussion
3.1. Maize Seedling Height and Growth Rate
- The heights of maize seedling measured at 5 DAP and 10 DAP are shown in Fig. 1, while the rates of growth (in cm day-1) are shown in Fig. 2. At 5 DAP, heights of maize seedlings averaged 12.89 cm to 13.89 cm and followed the trend SA> PLA>NF treatments (Fig. 1). At 10 DAP, however, maize seedling heights increased considerably, but the trend changed to PLA>NF>SA treatments (Fig. 1).As can be deciphered from Fig. 1, the percentage increase in maize plant height was in the trend NF = FA > SA. While NF and FA treatments respectively resulted in increments of 99.6% and 92.8%, from 5 DAP to 10 DAP, the increment recorded over the same period in SA treatment was 75.9%. The rates of growth of the maize seedlings from germination to 5 DAP followed similar trends with their heights at 5 DAP. However, even though the seedlings grew tall taller from 5-10 DAP, the rate of growth decreased by about 38.2% in NF and FA treatments and 49.3% in SA treatments (Fig. 2).
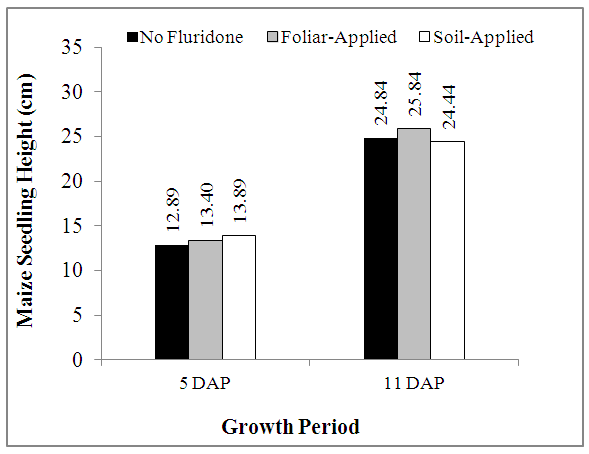 | Figure 1. Effect of Fluridone on height (cm) of maize seedlings |
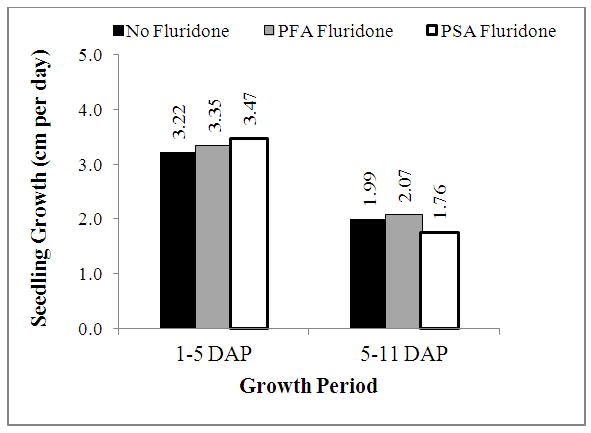 | Figure 2. Effect of Fluridone on growth rate of maize seedlings |
3.2. Maize Seedling Leaf Chlorophyll Content
- The results of in situ leaf chlorophyll measurements using the handheld atLEAF® Chlorophyll Meter is shown in Fig. 3. At 5 DAP, leaf chlorophyll contents followed a similar trend with the seedling height (Fig. 1), with the soil-applied Fluridone treatment have the highest count, while the NF treatment had the least.However, while the NF and FA treatments had higher readings, at 10 DAP, the Chlorophyll contents of maize grown on SA Fluridone treatment was significantly (P<0.05) reduced as compared to 5 DAP (Fig. 3).
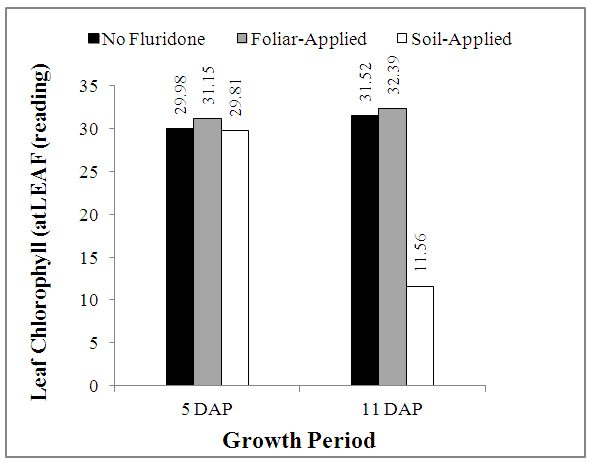 | Figure 3. Effect of Fluridone on chlorophyll content of m aize seedlings |
 | Plate 1. Effect of Fluridone on Maize Seedling Morphology |
 | Plate 2. Bleaching of Maize seedlings growing in SA treatment |
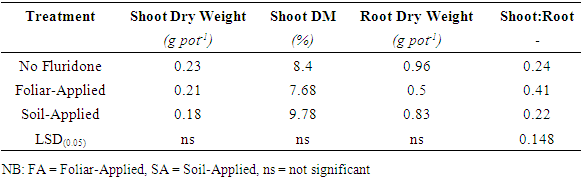
3.3. Effect of Fluridone of Maize Seedling Dry Weight
- The results of measurements of shoot and root dry weights, shoot dry matter contents and shoot to root ratio are shown in Table 1. The shoot dry weights of maize seedlings grown in different soil treatments with or without Fluridone did not differ significantly (P≤0.05), however, the trend was NF>SA>FA. In contrast, the dry matter contents of the maize seedlings varied significantly (P<0.05) with treatments (Table 1). The Application of Fluridone followed by prolonged drying decreased shoot dry matter content of maize seedlings by magnitudes of 40% in FA and 45% in SA treatments.
|
4. Conclusions
- It is discernible from this study that, after prolonged wet and dry cycles, the effect of residual fluridone affected the leaf chlorophyll content and resulted in wider shoot to root ration in maize seedlings. However, this effect was observed only in treatments where Fluridone was applied directly to soil. The use of Fluridone as foliar spray on one crop in one season, did not exhibit any adverse carryover effect on maize seedlings planted thereafter after a prolonged desiccation of the soil.This study clearly shows that the effect of Fluridone in coastal plains soils in the Niger Delta persists beyond the season in which it was applied; suggesting that Fluridone, as much as possible, be used for terrestrial herbicide control in coastal plains of the Niger Delta. However, considering the recent attention Fluridone is attracting for use to control recalcitrant herbicide-resistant weeds there is need to evaluate the residual effects of Fluridone using other crops other than cereals, e.g., legumes such as cowpea and peanut.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML