-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2018; 8(2): 53-58
doi:10.5923/j.re.20180802.05

Variation of Some Physicochemical Parameters to Improve the Properties of Clays of Madagascar: Case of Nitrates in Water
Andrianainarivelo Mahandrimanana, Ravelonandro Pierre Herve, Pelibitaka Litoo J. L. T.
Faculty of Sciences, Department (Mention) of Process and Industrial Ecology, University of Antananarivo, Madagascar
Correspondence to: Andrianainarivelo Mahandrimanana, Faculty of Sciences, Department (Mention) of Process and Industrial Ecology, University of Antananarivo, Madagascar.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Clay is a lamellar material made up of elementary layers (phyllosilicates) that pile, aggregate themselves, disaggregate according to the condition of the surrounding environment. This induced arrangement of the internal reactive interfaces, different from external surfaces, and confined spaces which are true nanoreactors. The small size of their layers of which the thickness is on a nanometric scale (millionth mm) confers on clays mineral all the remarkable properties exploited in many application that one daily mixes with since millenia. We applied one of these properties with the process of adsorption to highlight the elimination of certain pollutants in water. The processes of adsorption are the object of this study. They tend to develop quickly and are mainly used for the elimination of polluting compounds. This technique showed great capacities of depollution of waste waters. But, its performance and its effectiveness depend on the nature of the adsorbent. In practice, we will use the adsorbent white clay and green clay. This study makes it possible to determine the effect of the initial nitrate concentration, the effect of the pH and the effect of the load while adsorbing. The best results were got to 76.5% for white clay (WC) and 60% for green clay (GC) with 100mg/L-1. of Nitrate. Indeed, the good output of adsorption is obtained with acid pH for the two adsorbents with 200mg/L-1 of Nitrate. However, the percentages of retention of thenitrates ion on the adsorbents used vary 35% and 89% for white clay and 28% and 80% for green clay with 100mg/L-1 of Nitrate. The analysis is carried out by visible spectrometry U.V of type SHIMADZU U.V- 160A. The experiment shows that the white clay of the I.M.R.A is good adsorbent power that the green clay of Homéopharma.
Keywords: Nanotechnology, Water, Nitrates, Elimination of nitrates, Adsorption, Clay
Cite this paper: Andrianainarivelo Mahandrimanana, Ravelonandro Pierre Herve, Pelibitaka Litoo J. L. T., Variation of Some Physicochemical Parameters to Improve the Properties of Clays of Madagascar: Case of Nitrates in Water, Resources and Environment, Vol. 8 No. 2, 2018, pp. 53-58. doi: 10.5923/j.re.20180802.05.
Article Outline
1. Introduction
- Water, it is before all the source of the life, however it unequally remains distributed and too rare for an important part of humanity. The figures are there, disturbing: nearly 250 million human beings, representing 26 countries, miss water cruelly.In the countries in the process of development, 80% of the cases of pathologies listed find their origin in a water of poor quality. Diseases that one believed finished for a long time still prevail in Africa, which is intolerable at one time which is said industrialized. Generally, our waterways are polluted by urban, agricultural and industrial waste including of many toxic substances of synthesis which the natural processes do not succeed in breaking up. Even in tiny quality, some of these substances can be very detrimental.Pollution is not always visible. The water of a river or a lake can seem clean, even if it is still polluted. In the ground waters, on which the majority of the Malagasy people hope to be supplied, the pollutants are particularly difficult to detect. Moreover, the effects of pollution are not necessarily immediate; they can take years to be appeared. When pollution makes water unfit for consumption, with the leisure, agriculture and industry, it ends up returning the less aesthetic lakes and rivers. What is more serious, it is that when pollution destroyed the aquatic life and reduces its capacity of reproduction, it threatens possibly the human health. Nobody escapes the effects of the water pollution.One distinguishes several types of pollution, which can have a domestic origin, agricultural or industrial. Physical pollution destroys the transparency of water (presence of suspended matter), acts on its temperature (thermal pollution) or its radioactivity.Chemical pollution is due to undesirable substances (nitrates, phosphates) or dangerous (metals and others micro pollutants), which cause deep chemical imbalances (acidity, salinity) having biological effects. Most toxic products come from chemical industry, of the industry of metals, the agricultural activity and the discharges of domestic or industrial waste.Organic pollution of water, coming from domestic waste waters and agribusiness industries, causes an oxygen overconsumption (necessary to its degradation) and can result in the death of the aquatic life. It can also cause the appearance or the setting in solution of bad products (metals, ammonia, sulphides).Microbiological pollution in the water of the micro-organisms, of which some are pathogenic germs (virus, bacteria). The domestic hearths, the hospitals, the breedings and certain agribusiness industries reject germs likely to present a danger to health.We will be interested more particularly in chemical pollution because it is the most widespread pollution in the world. A molecule strongly used in the rural areas drew our attention, it is the nitrate which is polluting coming from several types of, in particular, agricultural and animalist waste. To try to solve the problem of water polluted by this input we used a cheap raw material which is also in the countryside, clay. Our topic of study is entitled “Variation of some physicochemical parameters to improve the activity of clays of Madagascar: case of nitrate in water”. In the first part, we will carry out a bibliographical study concerning clay and nitrate and the technique (adsorption) used to determine the variation of concentration of nitrate when it is in contact with clay. In the second part booked with the various physicochemical characterization of the precursors in particular the potassium nitrate solution, the clay solution and the adsorption of nitrate in clay. For that, we used two types of clay, green clay and white clay. The third part relates to the discussion of the results got compared to the bibliographical data. And finally, we will finish the article by a conclusion and the prospect for research around the polluting agent others that the nitrate.
2. Bibliographical Studies
- A. Clay [1, 2]1. Definition [3]It is a material of elementary layers (phyllosilicates) which pile up, incorporate themselves, disaggregate according to the condition of the surrounding environment. This induced arrangement of the internal reactive interfaces, different from external surfaces, and spaces confines which are true nanoreactors. The small size of their layers of which the thickness is on a nanometric scale (millionth mm) confers on clays mineral (aggregation layers in particles, then in aggregates on increasingly large scales) all the remarkable properties exploited in many application that one daily mixes with since millenia. 2. Structure of clays minerals [4]The phyllosilicates are hydrated aluminium silicates corresponding to the formula (Si2O5)n. One can also consider that their structure is a combination of two fundamental structural layers formed by the assembly of the ion of the types O2, OH- and some cation: Al3+, Mg2+, and Si4+.The first unit is represented by tetrahedrons. Each tetrahedron is consisted a superposition of a hexagonal layer and a compact layer. The hexagonal layer is formed by six ion of oxygen laid out in hexagon and thus form cavities called “hexagonal cavities”.The superposition of these two layers generates tetrahedrons formed exclusively by all three ion O2 which clearly seems component of the called hexagonal layer “basal layer”. These three oxygen ion cap an ion O2 of the sub-base called “apical layer”. In the center of each formed tetrahedron exists a vacuum occupied by a cation. Generally Si4+ (R=0,41 Å) can be replaced by other ion Al3+, K+, Na+, Mg2+, or Fe3+ (R=0,50Å)The second unit is represented by the octahedral ones. Each octahedral is formed by a superposition of two compact layers of ion O2 and OH- and octahedral form what is called layer. The tops of each octahedral are occupied by ion of oxygen or hydroxide such as the whole of three ion is registered in an equilateral triangle pertaining to the roadbase. This last cap a similar structure belonging to the roadbase and the sub-base but laid out of such way that the top of the lower triangle is opposed higher triangle. In the center of each tetrahedron exists a cavity called “tetrahedral cavity”. Each cavity tetrahedron occupies more often Si4+. Dans each cavity octahedral contains a cation like Al3+, Mg2+, Fe3+, Fe2+, Ni2+, Li+ but Al3+ and Mg2+ are most frequent.Thus, the cavity inside a tetrahedron is smaller than the octahedral cavity. If the cation which occupy the cavities are bivalent for example: ion Mg2+, all the cavities are occupied and of the phyllosilicates are known as “trioctaedric”. On the other hand if the octahedral cavities contain the ion Al3+ trivalent, two thirds of the cavities are only occupied and the corresponding phyllosilicates are called “octahedral”. The ion O2- and OH- are charged negatively then they will tend to be pushed back between themselves. Thus the positive loads of the cation placed in the tetrahedral or octahedral cavities will balance them and ensure the stability of the unit.
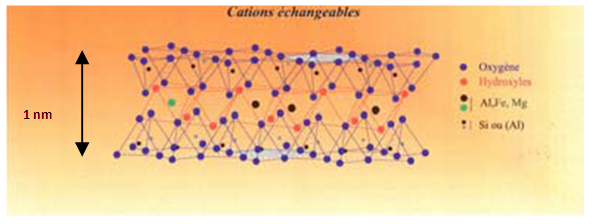 | Figure 1. Structure of the clay layers1 |
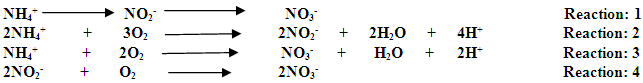 2. Origin of nitrates in water [1, 7]In water nitrates are naturally present at amounts varying according to the seasons and the places. The concentration of nitrates in surface water and underground are generally expressed in milligrams per liter. The main sources of nitrates in water are: - Wastes industrial and domestic;- Rejection of effluents of breeding;- Food of surface waters by polluted table cloths;- Chemical fertilizers employed to improve the growth of the cultures;- Waste of animal origin coming from barns and places of storage of manure; - Waste of human origin coming from fields of purification, or septic tanks or no tight tanks of retention; - Animal manure C. GENERAL INFORMATION ON ADSORPTION1. Definition of adsorptionAdsorption is the fixing of a gas or a liquid (adsorbate) on the surface of a solid (adsorbent). It occurs with the interface of an adsorbing adsorbate 2.The solid which is the seat of this adsorption is called adsorbent while the liquid or the gas which undergoes adsorption is called adsorbate. Figure 2 below watches the phenomenon of adsorption.
2. Origin of nitrates in water [1, 7]In water nitrates are naturally present at amounts varying according to the seasons and the places. The concentration of nitrates in surface water and underground are generally expressed in milligrams per liter. The main sources of nitrates in water are: - Wastes industrial and domestic;- Rejection of effluents of breeding;- Food of surface waters by polluted table cloths;- Chemical fertilizers employed to improve the growth of the cultures;- Waste of animal origin coming from barns and places of storage of manure; - Waste of human origin coming from fields of purification, or septic tanks or no tight tanks of retention; - Animal manure C. GENERAL INFORMATION ON ADSORPTION1. Definition of adsorptionAdsorption is the fixing of a gas or a liquid (adsorbate) on the surface of a solid (adsorbent). It occurs with the interface of an adsorbing adsorbate 2.The solid which is the seat of this adsorption is called adsorbent while the liquid or the gas which undergoes adsorption is called adsorbate. Figure 2 below watches the phenomenon of adsorption. | Figure 2. Phenomenon of adsorption |
3. Various Kinds of Adsorption
- According to the nature of the forces which retain the molecule adsorbed on the surface of the solid. There exist two types of adsorption: chemisorption, and physical adsorption.
3.1. Chemical Adsorption or Chemisorption
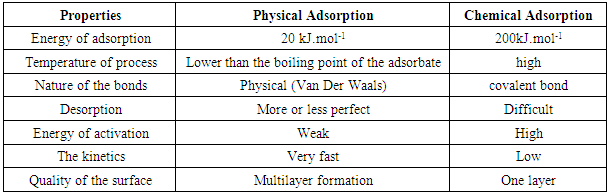
- Chemical adsorption is generally irreversible. It brings into play one or more covalent bonds or ionic between the adsorbate and the adsorbent. This kind of adsorption brings into play energies of high attraction. The heat of adsorption is relatively high. It is of the order 200 KJ.mol-1 [8]. The various chemical bonds in chemisorption are:- The purely ionic bond of the atoms donors or acceptors of electrons, - The covalent bond or metal,
3.2. Physical Adsorption
- Physical absorption is characterized by the forces between the molecules of liquid or gas and solid. These forces are of type Van Der Waals. It is reversible. Put energy concerned is weak, it is of the order 20 KJ.mol-1 [9].Chemisorption and physical absorption are differed by their energy, their temperature of process, their nature of connection and their kinetics. Table 1 represents these differences.
|
3.3. Factors Influencing Adsorption [1]
- The factors influencing adsorption are the temperature, the load of the adsorbent, the nature of the adsorbate and specific surface.- Temperature Adsorption is an exothermic process and consequently its unfolding must be favored at low temperature.- Charge of the adsorbentThe capacity, the speed and the heat of adsorption decrease when the load (adsorbed fraction) of the adsorbent increases.- Nature of the adsorbateTo have a good adsorption, it is necessary that there is initially an affinity between the solid and the aqueous solution. In general, the polar solids, adsorb other polar bodies preferentially. On the other hand the nonpolar solids, adsorb nonpolar substances preferentially. Consequently, affinity for the substrate grows with the molecular mass of the adsorbate.- Specific Surface [10, 11, 12]Specific surface gives primarily the characterization of the solids and porous materials. More, it is large, more adsorption is better.
4. Results of the Tests of Adsorption
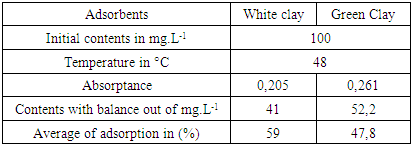
- A Experimental methods We chose potassium Nitrate like adsorbent and we tested the adsorbing power of two clay samples, the white clay of the IMRA and the green clay of Homeopharma. The adsorption of nitrate is analyzed with a UV-visible spectrophotometer. The maximum wavelength of detection of nitrates is of 220 Nm. We tried to vary various parameters such as the nitrate concentration, the pH, the load while adsorbing and the temperature of adsorption in order to optimize the requirements to have a maximum adsorption.1. Effect of the initial nitrate concentration The outputs of retention of thenitrates ion were given for the two clay samples: white clays and green clays under the same operating condition, namely: 5g of clay per liter of distilled water and the initial nitrate concentration of 100 mg. L-1, 350 mg. L-1 and 700 mg. L-1. The tests of adsorption were carried out at temperature 25°C under agitation during 60 mn [13]. It should be noticed that as the output of adsorption decreases, the initial concentration of nitrates increases with the same quantity of adsorbent (20.029% for white clay and 13.914% for green clay with the same initial concentration).
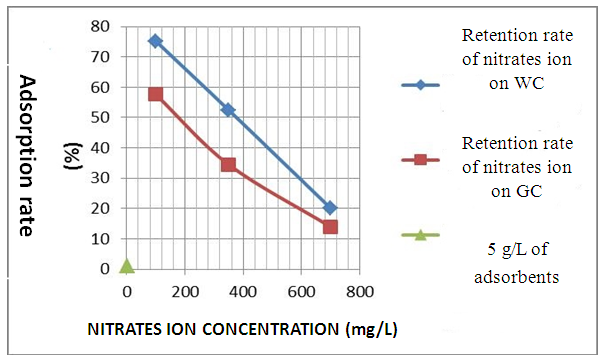 | Figure 3. Average output according to the concentration in N03- ion at room temperature (25°C) with 5 g.L-1 of adsorbent |
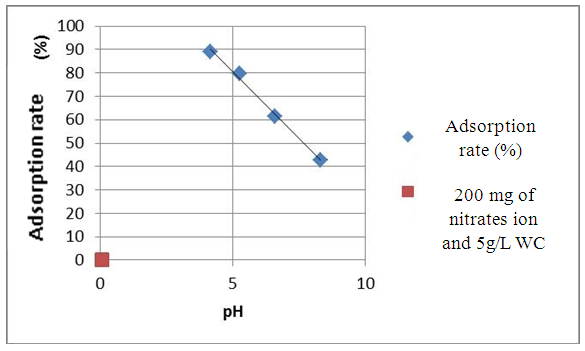 | Figure 4. Effect of the pH on the adsorption of the nitrates ion at T=25°C for 5g.L-1 with white clay and 200 mg. L-1 of NO3- |
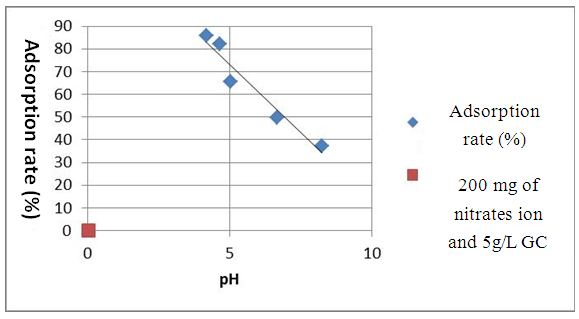 | Figure 5. Effect of the pH on the adsorption of the nitrates ion at T=25°C for 5g.L-1 with green clay and 200 mg. L-1 of NO3- |
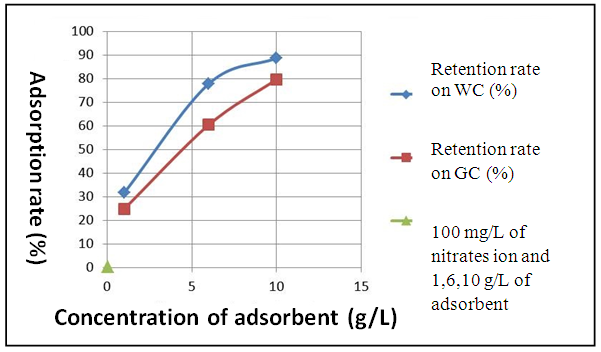 | Figure 6. Output of adsorption of nitrate according to the concentration while adsorbing in T= 25°C |
|
5. Discussion
- White clay is also called Kaolin. It has a good adsorbent power. It is a clay of the Smectite family. For the smectite, the capacity of fixing is very important, thanks to the loads (negative of the layers, positive between the layers) and on the considerable specific surface of 100 m2 for one gram. These interaction can be done in three different sites:- in the layers for the simple ion, between the layers for the molecules punts or small, with the periphery for the macromolecules.Green clay belongs to the family of the illite. Its quality is lower than those of smectite. It has a good power of absorption but a small power of adsorption [14].According to the results which we got while varying some physicochemical parameters, clays have a power adsorbing for the nitrate molecules. We chose this pollutant because it is most widespread as well in urban environment as rural. Variation of the initial nitrate concentration of 100 mg. L-1, 350 mg. L-1 and 700 mg. L-1 for the same quantity of clay (5g.L-1) showed that white clay has a power adsorbing higher than that of green clay. Indeed, the highest output of adsorption of the nitrates ion (75.2%) is observed on white clay, for an initial nitrate concentration corresponding to 100 mg. L-1 while the proportion does not exceed the 57% for green clay for the same initial nitrate concentration. In agreement with BOUALLA [15], it should be noted that the agitation of the solution should not exceed the 60 minutes to have a maximum output. The increase in the pH supports the adsorption of the nitrates ion on clays because one eliminates the ion OH- which return in competition on fixing. When one increases the concentration while adsorbing, one observes a growth of the output of adsorption up to 10 g.L-1.
6. Conclusions
- This work aims to reduce the quantity of nitrates present in water. The adapted method is the adsorption of white clay of the I.M.R.A and green clay solution of Homéopharma. To be able to conclude it, the interest of this study concentrated on the effect of the initial concentration innitrates ion, the pH and the load while adsorbing. The tests of adsorption were analyzed with visible spectrometry U.V. The interpretation and the confrontation of the results got through this proportioning, made it possible to draw the following conclusion: - The highest output of adsorption of the nitrates ion (75.2%) is observed on white clay and to 57.6% on green clay, for an initial nitrate concentration corresponding to 100 mg. L-1. It decreases when the initial concentration of nitrates increases with the load while adsorbing retained. - The pH is an important parameter for adsorption. An increase in the pH can decrease the capacity of fixing of white clay and green clay of Madagascar. Adsorption is ineffective for a basic pH. On the other hand, it is effective for a pH lower or equal to 6.6. The best output of adsorption is observed with strongly acid pH.- The output of adsorption of the nitrates ion is almost identical for two clays used. The percentages of retention of the nitrates ion on the adsorbents used vary from 31.6% to 88.6% for white clay and from 24.8% to 79.6% for green clay with 100 mg. L-1 of Nitrate. The maximum values of adsorption are respectively of 88.6% and 79.6% for white clay and green clay. - The output of adsorption of nitrates decreases when the temperature thus increases adsorption is ineffective with high temperature. Adsorption is a very effective method to retain pollution present in water, in particular thenitrates ion. The experiment shows that white clay is a good adsorbing compared to green clay (to be able adsorbent fairly). All in all, the white clay of the I.M.R.A and the green clay of Homéopharma produce a positive effect to reduce the nitrate rate present in water.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML