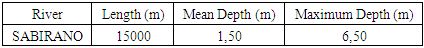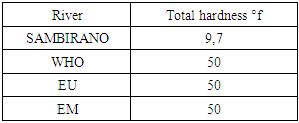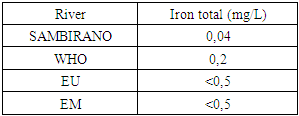Razafitsiferana Théophile, Bruno Razanamparany, Mihasina Rabesiaka, Andrianainarivelo Mahandrimanana
University of Antsiranana, Faculty of Sciences, Chemical Sciences, Mineral Chemistry, Madagascar
Correspondence to: Razafitsiferana Théophile, University of Antsiranana, Faculty of Sciences, Chemical Sciences, Mineral Chemistry, Madagascar.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The district of AMBANJA is south of DIEGO SUEREZ, it is among the five districts that form the region DIANA. The SAMBIRANO River crosses in the south of the city of AMBANJA on the district named AMBAHIBO. The objective of the work is the determination of the concentration of physico-chemical parameters and the microbiological analysis of the water of this river. The measured temperature is 19.3°C per thermometer, the turbidity 11.7 NTU per turbidimeter, the pH 7.58 per pH meter and the conductivity 73.8μS / cm per conductivity meter. The physical parameters the temperature, the pH and the conductivity are admissible to the norms of the drinking water, on the other hand the turbidity is outside the value required for the international standards. The mineralization measured is 68mg / L, total hardness is 9.7 °f, calcic hardness 4.5 °f, the TAC is 3.2 °f, the Calcium is 18mg / L, the Magnesium is 12.64 mg / L, the Bicarbonates is 39.04mg / L, Organic matter is 1.62mg / L, the ammonium is 0.14mg / L, the Total iron is 0.04mg / L, the chlorides is 3, 55mg / L, Sulfates 3.17mg / L, Nitrates is 7.39mg / L, Nitrates 0.03mg / L and Sodium is 2.3mg / L, the concentrations of the chemical parameters are eligible for international standards for l drinking water despite the insufficiency of some concentrations for sodium, calcium and magnesium. Coliform bacteria is greater than 1440 MPN / 100ml, Escherichia coli is 4 NPP / 100mL and intestinal enterococci is 1.5 NPP / 100mL, microbiological parameters are excluded to the values required for drinking water standards, it is ie river water is microbial.
Keywords:
Water, Physicochemical and microbiological parameters
Cite this paper: Razafitsiferana Théophile, Bruno Razanamparany, Mihasina Rabesiaka, Andrianainarivelo Mahandrimanana, Analysis Physico-Chemical Parameters of the Sambirano River Water in the Ambanja District Located in the Diana Region, Resources and Environment, Vol. 8 No. 2, 2018, pp. 43-52. doi: 10.5923/j.re.20180802.04.
1. Introduction
The District of AMBANJA is among the five districts which form the Region of DIANA; it is in the South of Diego Suarez, the population its account 200 245 it is distributed in Ten (10) Fonkotany. The following table gives the numbers of populations that the RIVIERE uses as drinking water.Table 1. Numbers of populations use the river as drinking water
 |
| |
|
The characteristics of this river is given by the following table:Table 2. Characteristics of the SAMBIRANO River
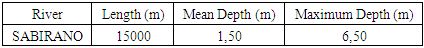 |
| |
|
This study has four parts, the first part is the bibliographic synthesis, the second part is the measurement results for the physicochemical parameters and the microbiological analysis, the third part the interpretation of the results, and the fourth part is the method for disinfection, we will end with the conclusion.
2. Bibliographic Synthesis
Three possible structures for water, solid, liquid or gaseous. The chemical separation of water is the dissociation into H+ and an OH-.Water contains several organic materials; it has dissolved form in water. Metals can exist in water, it presents in the form of a trace. Pollution that exists in the water, too high concentrations of pollution in the water causes a microbe.
3. Quality Standard
For drinking water reference is made by the standard trios: WHO, European Union (U E) and the Malagasy State (E.M).1 - Recommendation of the WHO 2 - Recommendation of EU 3 - Recommendation of the EM Table 3. Recommendation of the WHO
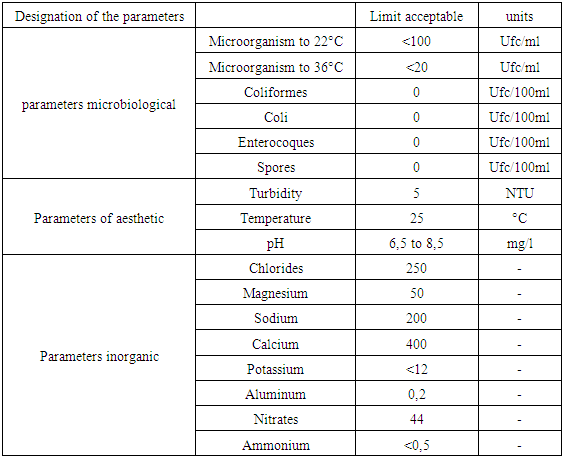 |
| |
|
Table 4. Recommendation of the EU
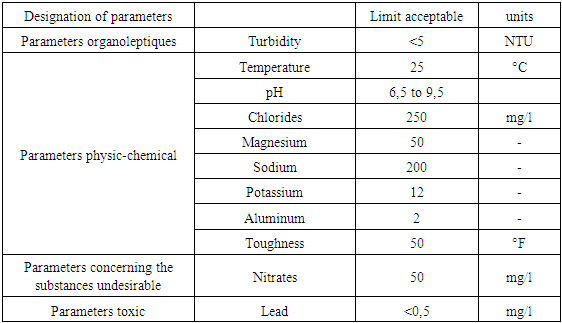 |
| |
|
Table 5. Recommendation of the EM
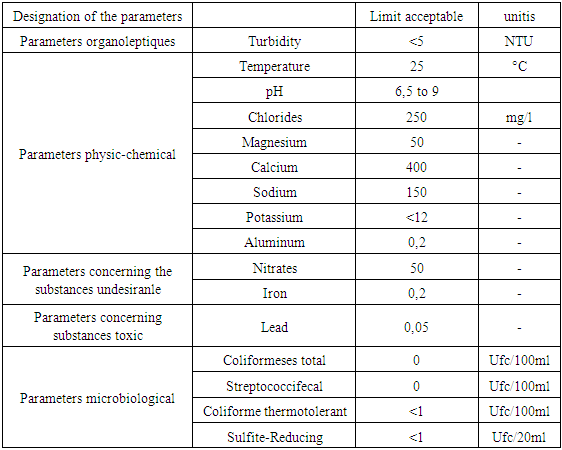 |
| |
|
4. Parameters of Analysis
The pH to know the water is basic, or acid and neutral, the turbidity to know the transparency of the water, the conductivity to know the quality of the salt dissolved in the water, the organic matter makes it possible to estimate the quantity of organic matter in water such as BOD, BOD5 and COD.The T A and TAC is the basic salt content, that is, the determination of the OH- ion concentration in the water.Nitrate assays is the determination of the concentration of nitrates in water, determining the concentration of calcium and magnesium for the existence of total hardness, the concentration of iron that exists in water is known by the dosage of total iron, the concentration of ammonium in water indicates the existence of pollution.The average concentration of calcium, sodium and magnesium play a very important role for the daily life of humans.Microbiological analysis these very important for drinking water.Results of MeasuresI – Physical parameters1. TemperatureTable 6. Represents of the temperature
 |
| |
|
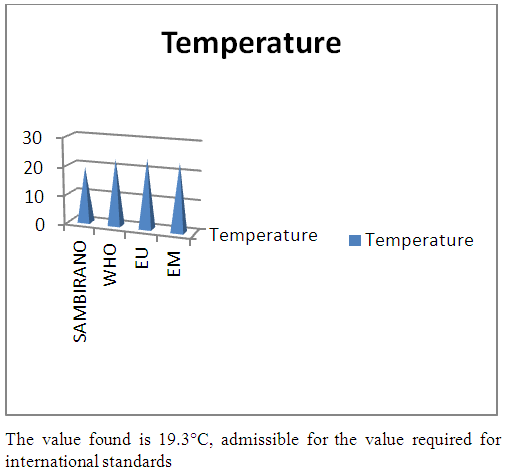 | Figure 1. Temperature measurement |
2. TurbidityTable 7. Represents of the turbidity
 |
| |
|
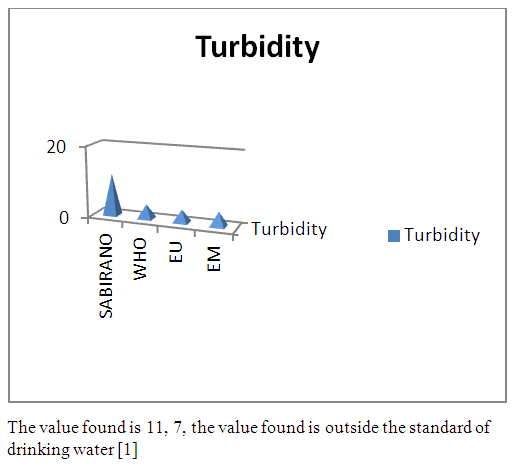 | Figure 2. Turbidity measurement |
3 – pHTable 8. Represents of pH
 |
| |
|
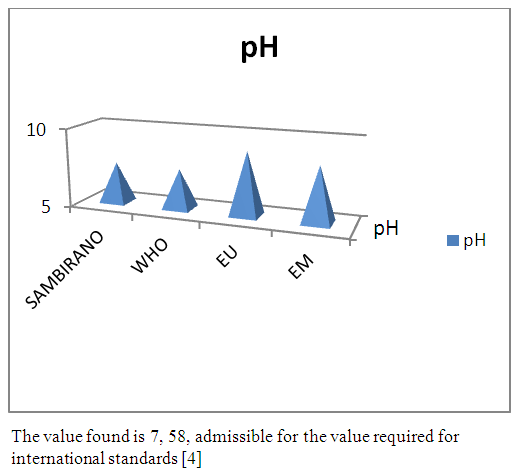 | Figure 3. pH measurement |
4 - ConductivityTable 9. Represents of conductivity
 |
| |
|
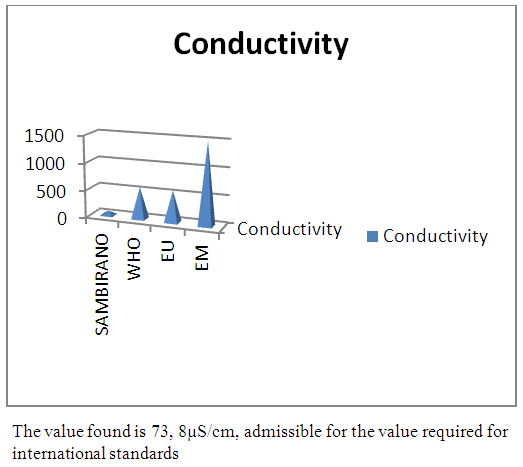 | Figure 4. Conductivity measurement |
II – CHEMICAL PARAMETERS1 – Mineralization Table 10. Represents of mineralization
 |
| |
|
 | Figure 5. Mineralization measurement |
2 – Total hardnessTable 11. Represents of Total hardness
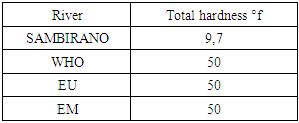 |
| |
|
 | Figure 6. Total hardness measurement |
3 - Calcium hardness Table 12. Represents of Calcium hardness
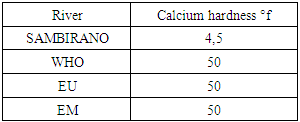 |
| |
|
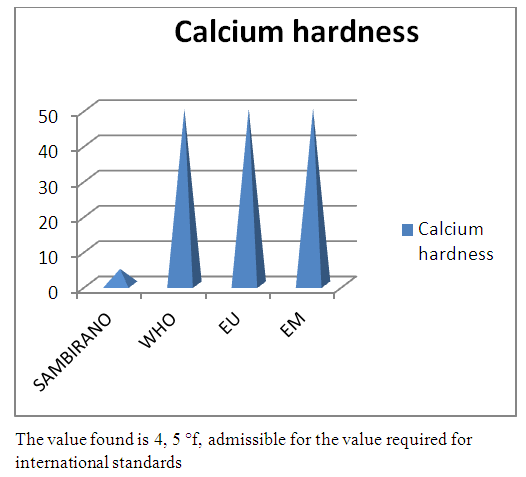 | Figure 7. Calcium hardness measurement |
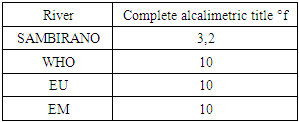
4 - Complete alcalimetric title (T A C)Table 13. Represents of complete alcalimetric title
 |
| |
|
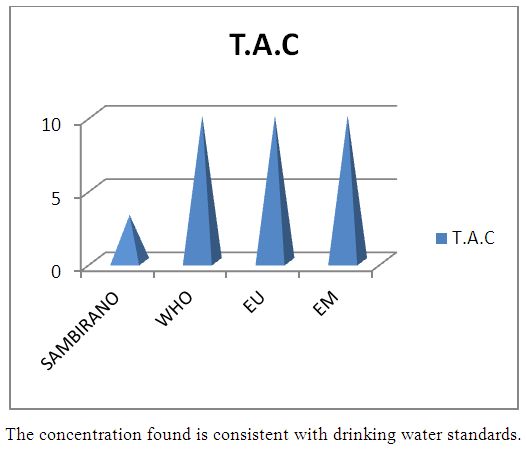 | Figure 8. TAC measurement |
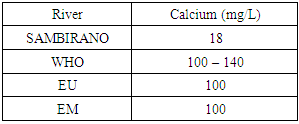
5 – CalciumTable 14. Represents of calcium
 |
| |
|
 | Figure 9. Calcium measurement |
6 – MagnesiumTable 15. Represents of magnesium
 |
| |
|
 | Figure 10. Magnesium measurement |
7 – SodiumTable 16. Represents of Sodium
 |
| |
|
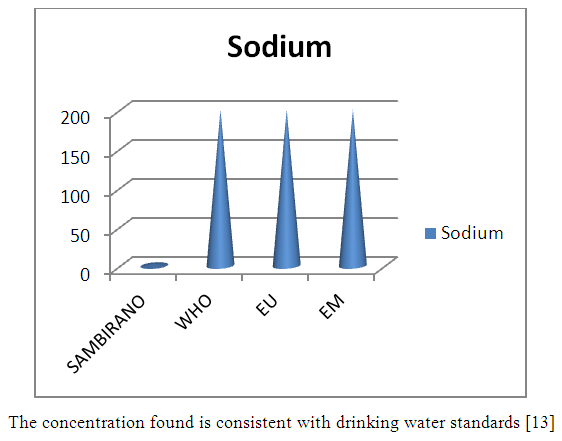 | Figure 11. Sodium measurement |
8 - Organic materialTable 17. Represents of Organic material
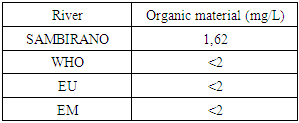 |
| |
|
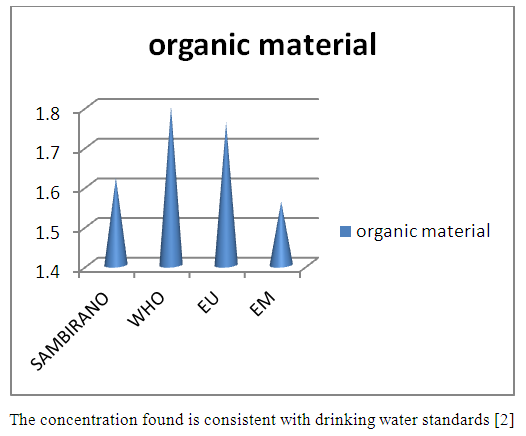 | Figure 12. Organic material measurement |
9 – AmmoniumTable 18. Represents of ammonium
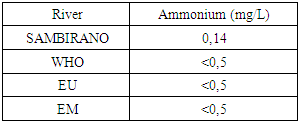 |
| |
|
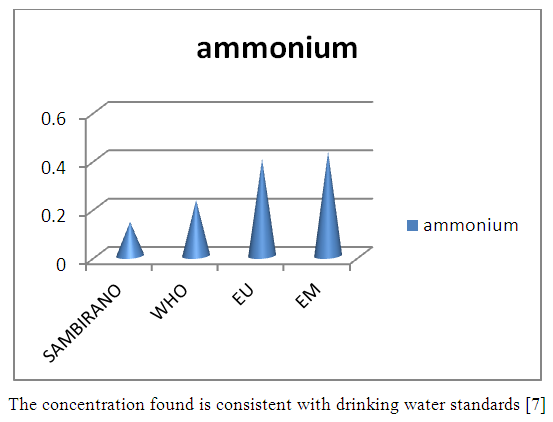 | Figure 13. Ammonium measurement |
10 – Iron totalTable 19. Represents of Iron total
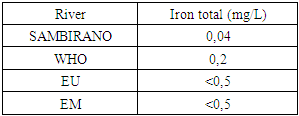 |
| |
|
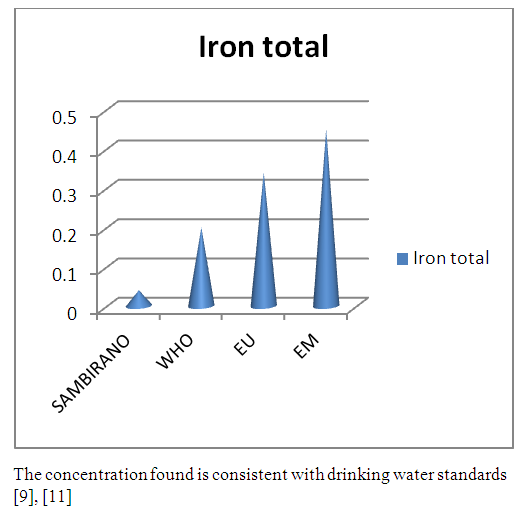 | Figure 14. Iron total measurement |
11 – ChloridesTable 20. Represents of Chlorides
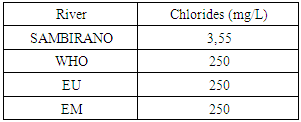 |
| |
|
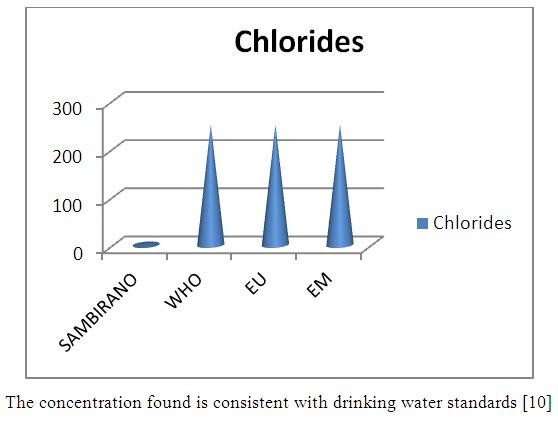 | Figure 15. Chlorides measurement |
12- NitratesTable 21. Represents of Nitrates
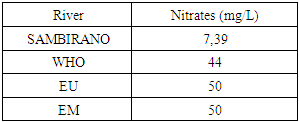 |
| |
|
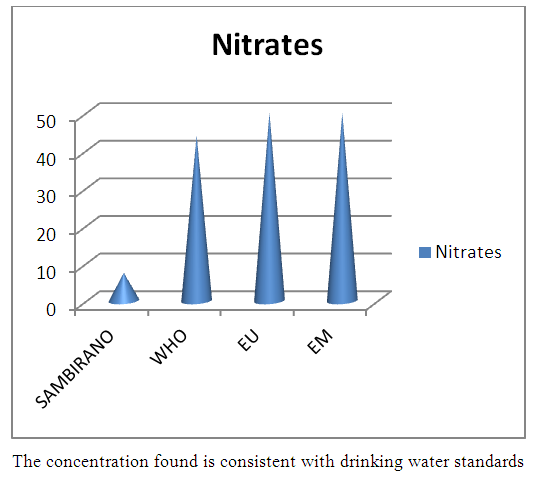 | Figure 16. Nitrates measurement |
13 – CopperTable 22. Represents of Copper
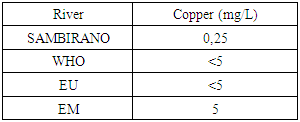 |
| |
|
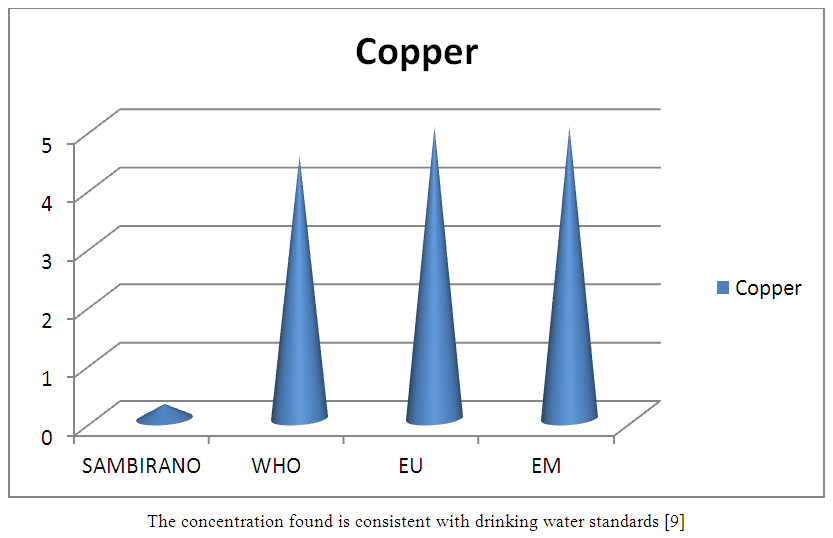 | Figure 17. Copper measurement |
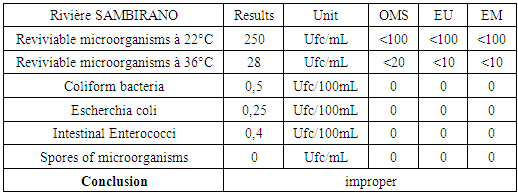
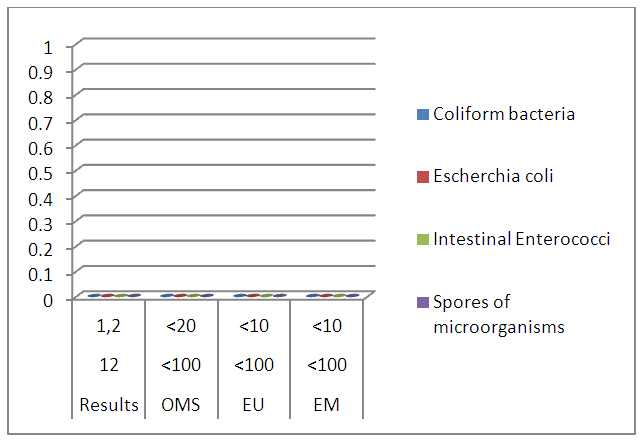
III – MICROBIOLOGICAL ANALYSISTable 23. Measurement results of the microbiological
 |
| |
|
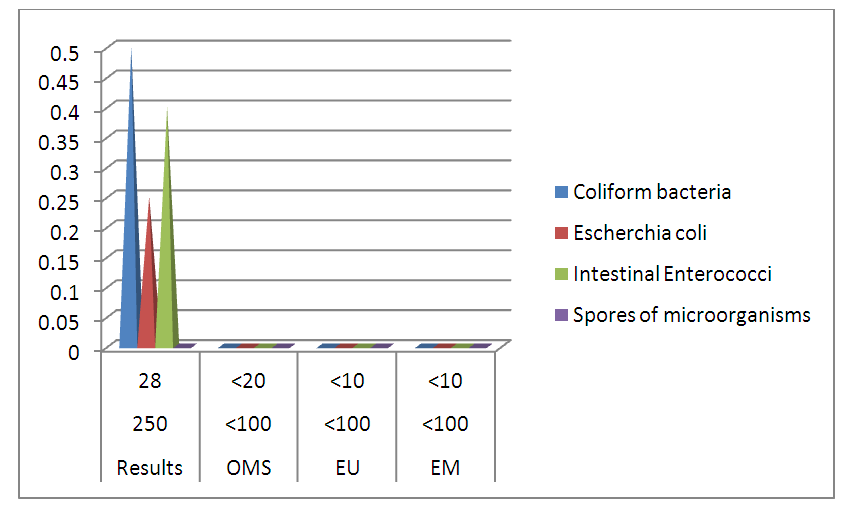 | Figure 18. Microbiological analysis results |
The table shows that the water of the river is microbial, so it must be disinfected. [14], [15].For the disinfected one uses the method by chlorination.
5. Method of Treatments
We performed a chlorine demand test of this sample; this test provides an optimal rate of calcium hypochlorite [Ca(Cl)2] required to have disinfection.The result of the chlorine demand is given by the following table N° 24
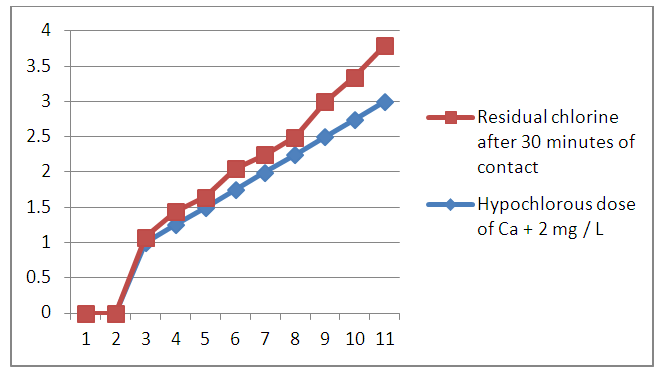 | Figure 19. The result of the chlorine demand is given by the following |
Interpretation of the curve:from 1 to 1.75 mg / L: chlorine in the form of amine compoundsfrom 1.75 to 2 mg / L: destruction of chloramines by an increased dose of chlorine.from 2 to 3mg / L: progressive increase of Cl2the optimal dose of calcium hypochlorite used is between 2 to 3 mg / L to avoid any risk of contamination, during the rainy season.Table 25. Post-treatment results for microbiological analysis
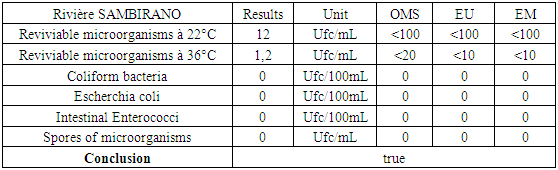 |
| |
|
 | Figure 20. Microbiological analysis results after treatment |
6. Interpretation of Results
- Physical parametersThe temperature found is 19.3°C, below 25°C, it is eligible for international standards for drinking water, the turbidity is 11.7 NTU, greater than 5 NTU so outside the value required for the standard of potability, the pH is 7.58 it between 6.5 and 8.5 admissible to the potability standard and the conductivity is 73.8μS / cm between 180 and 1000 μS / cm meets the standard required for standards international. The physical parameters therefore perfectly meets the potability norm, in particular the turbidity is excluded for the required values the river water SAMBIRANO is very hard.- Chemical parametersThe river water of SAMBIRANO is mineralized because the concentration found is equal to 68mg / L, it is very close to the value required for international standards.The total hardness is 9.7 °f and the hardness Calcique is 4.5 °f so the calcium and magnesium level in the SAMBIRANO River water is acceptable for the potability standard.Organic matter is 1.6mg / L, ammonium is 0.14mg / L they are acceptable for international standards.The calcium is 18mg / L, the magnesium is 12.64mg / L, they are eligible for the standards required for drinking water because the calcium is 400mg / L the magnesium is 50mg / L.The sodium is 2,3mg / L the required value is 200mg / L it qualifies unwillingly the insufficiency of this found sodium concentration.The T A C is 3.2 °f the required standard is 11 °f so it is acceptable for drinking water.The Nitrate is 7.39mg / L the required value is 44mg / L on average, therefore eligible for the standard of drinking water. The concentration of chloride found is 3.55mg / L, it is far below the value required for the standard of drinking water because the required standard is 250mg / L.Chemical parameters almost acceptable to international standards, notwithstanding some insufficiency of the concentration found as calcium, chloride, magnesium and sodium.- Microbiological analysisFor the microbiological analysis of the water of the SAMBIRANO River, the results found are excluded to international standards for drinking water, so the water is microbial.I propose you a method for the disinfection of this water is by the method of CHLORATION with the optimal value and without risks meme for the period of rain one uses between 2 and 3,5mg / L of calcium hypochlorite.
7. Conclusions
In conclusion the results of measurement for the physical parameters of the river SAMBIRANO: the pH = 7,58, temperature = 19,3°C and the conductivity is 73μS / cm are standard for drinking water, but the value of the turbidity found is very large, ie equal to 11.7NTU, this value is excluded for the standard required for drinking water because limit value is less than 5NTU. So the SAMBIRANO river water can use but it is necessary to treat the turbidity before using because the water is very hard.For the chemical parameters are in norms but their found concentrations are very low, respectively the concentrations of chloride 3,55mg / L the required value is 250mg / L, sodium 2,3mg / L the required value is 200mg / L, magnesium 12, 64mg / l the required value is 50mg / Let the Calcium 18mg / L the found value is 400mg / L. The water is drinkable despite some concentrations of very important parameters for drinking water is insufficient so it is necessary to increase their concentration before being used, I propose the method of demineralization that is to say the increase in the rate of insufficient parameters for the river water of SAMBIRANO.For microbiological parameters the microbiological parameters that we will study in the SAMBIRANO river does not answer exactly the norms required for the international norms for the drinking water that is to say the water is microbial, that is why my work is used the method by chlorination to disinfect these microbes.
References
| [1] | V S. HART et al. (1992). An analysis of low-level turbidity measurements. J. Amer, Water Works Assoc. 84, (12), p. 40. |
| [2] | M. MONTGOMERY (1967). Determination of dissolved oxygen by the Winker method. J. Appl. Chem. |
| [3] | C. RICHARD, N. VAN Cu (1961). Relation entre la résistivité d’une eau et son taux de mineralization. L’eau, 1, p. 22-24. |
| [4] | G. VIVIN (février 1957). Mesure et regulation of pH, Génie Chimique, 37. |
| [5] | T. E. LARSON and L. M. HENLEY (1955). Determination of low alkalinity or acidity in water. Anal. Chem. 27: 851. |
| [6] | F. MORAN (2002). Chauffage, climatisation, installation sanitaires: traitement des eaux. EDIPA Editions Parisiennes, Chaud-Froid-Plomberie, Paris. |
| [7] | R. L. BOOTH and R.F. THOMAS (1973). Selective electrode determination of ammonia in water and wasters. Environ. Sci. Technol. 7: 523. |
| [8] | J. RODIER, C, GRAUDE (1952). Détermination de la dureté dans les eaux par la method au complexon III. Bull. Institut d’Hygiène du Maroc, N, S. XII. (3-4), p. 275. |
| [9] | Y U.G. VLASOV et al. (1997). Flow-injection analysis of nautral waters with a chloride-sectctive electrode, Journal of Analytical Chemestry, 52; 81. |
| [10] | D. BLAIR and H. DIEL (1961). Bathophenanthroline disulfonic acid and bathocuproine disulfonic acid, waters soluble reagents for iron and copper, Talanta, 7: 163. |
| [11] | M. T. DOIG and D. F. MARTIN (1971). Effect of humic acids of iron analyses in natural water. Water Res, 5: 589. |
| [12] | A. J. DOWNARD et al. (1992). Amperometric techniques in flow-injection analysis. Determination of magnesium in sera and natural waters, Analytica Chimica Acta, 249: 469. |
| [13] | A. M. URE and R.L MITCELL (1975). Sodium, Potassium., Calcium Flame emission and atomic absorption spectrometry, Dekker, New York, N.Y.9: 823. |
| [14] | H. LECLERC, R.. BUTTAUX (1961). Survie des spreptocoques fécauxdans les échantillons d’eaux stockées à +4°C. Ann Inst. Pasteur, Lille. 12, p. 211. |
| [15] | J.H. STANDRIDGE, D.J. LESAR (1978). Comparaison of hour and twenty four hour refrigerated storage of non potable water for fecal coliform analysis Appled Environmental Microbiology, 34, p. 398. |























 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML