-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2018; 8(2): 38-42
doi:10.5923/j.re.20180802.03

Residual Fluridone in Humid Tropical Soils: Carryover Effects on Germination and Seedling Growth of Maize (Zea mays L.)
Elsie I. Hamadina 1, Mohammed K. Hamadina 2
1Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria
2Department of Soil Science, Faculty of Agriculture, University of Abuja, Abuja, Nigeria
Correspondence to: Mohammed K. Hamadina , Department of Soil Science, Faculty of Agriculture, University of Abuja, Abuja, Nigeria.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Fluridone is an herbicide that is non-toxic to animals, and it is even being explored for use as drugs for humans. Fluridone helps to release dormancy in plants, with successes achieved for very dormant plants such as yam and Allanblackia. However, little is known about the residual effects of Fluridone in soil, and there is a dearth of information on the carryover effects of soil applied Fluridone on crops. The carryover effects of Fluridone were in a 2 x 3 factorial experiment (2 maize varieties and 3 Fluridone treatments); using Sammaz-40 and Oba Super-6 as test plants sowed in each of these treatments: 1. No Fluridone (NF), 2. Previous Soil-Applied Fluridone (PSAF), and 3. Previous Foliar-Applied Fluridone (PFAF). The Fluridone treatments were applied during the growth cycle of a previous yam crop; soil from that experiment was used in this trial. Three days after planting (DAP) more than half of the Oba-Super 6 hybrid maize seeds planted had emerged, as compared to 13% of Sammaz-40 seeds. In PSAF treatment, 83% of Oba-Super 6 plants had emerged by 6 DAP, as compared to 73.3% and 43.3% for PFAF and NF respectively. The PSAF treatment reduced leaf area and chlorophyll contents, while Sammaz 40 had significantly higher leaf area than that of Oba-Super 6. The carryover effects of soil-applied Fluridone are deleterious to maize seedlings planted after a previous Fluridone application.
Keywords: Residual Toxicity, Fluridone, Maize, Chlorophyll, Herbicide
Cite this paper: Elsie I. Hamadina , Mohammed K. Hamadina , Residual Fluridone in Humid Tropical Soils: Carryover Effects on Germination and Seedling Growth of Maize (Zea mays L.), Resources and Environment, Vol. 8 No. 2, 2018, pp. 38-42. doi: 10.5923/j.re.20180802.03.
Article Outline
1. Introduction
- Any chemical that is utilized in agriculture should not only be effective in terms of the intended intervention, but such chemical must not be toxic to living organisms, and should not have negative impacts on the environment [1]. Fluridone contains 41.7% 1-methyl-3-phenyl-5-[3-(trifluoromethyl) phenyl] pyridine-4-one, with chemical formula C19H14F3NO, is a known herbicide that has been used to effectively control aquatic weeds [2]. Reports show that Fluridone is not toxic to animals at the recommended concentrations for weed control, and no deleterious effects have been reported, at least for the aquatic ecosystem where it is mostly used [2].Although Fluridone is basically utilized as an aquatic herbicide, it has gained recent attention for other uses, due to its mode of action, as potential chemical for manipulating physiological processes in living things [2-4]. For instance, promising results have been reported for dormancy release in both domesticated and wild plants such as Dioscorea spp [4] and Allanblakia floribunda [5, 6] respectively. Fluridone is also being exploited for possible use as anti inflammatory drug in mammals [2].However, studies on Fluridone’s persistence in soils are limited and the results are inconsistent: its half-life in soil has been reported to vary from less than 20 days to greater than 300 days [7]. Nevertheless, it is reported that soil parameters such as pH, clay content and organic matter affect the persistence of Fluridone in soils [8, 9]. Similarly, studies on the residual effects of soil applied Fluridone on subsequent plant are scarce, as most studies reported only the direct effects on target plants, but not on subsequent or non-target plants. Several soil entry pathways are discernible for Fluridone, including application in irrigation water, direct soil application as pre-emergence herbicide, indirectly via plant exudates from plants that had foliar-applied Fluridone, and as aerosol fallout when spraying Fluridone solution [10].Fluridone is reported to control weeds by preventing biosynthesis of carotene, and abscisic acid (ABA), and the eventual photolysis of leaf chlorophyll [11]. Chlorophyll level is an important index of crop health and chlorophyll content of leaves is used to assess plant nutrient status, especially nitrogen, and as a diagnostic tool for plant diseases. Leaf chlorophyll measurements have been reported to be a useful tool in assessing the effectiveness of herbicides that destroy chlorophyll [12]. Therefore, chlorophyll contents of leaves can be used to assess the effects of Fluridone. Chlorophyll measurements in plants can be achieved using several methods that can be categorized as: destructive and non-destructive; each with its merits and challenges. However, the choice of methods depends on the objectives for the measurement, costs and the technology available. Non-destructive measurements have the advantage of speed of measurement, relative accuracy and repeatability. However, it often requires expensive equipment, which may not be available locally.This study was conducted to test whether Fluridone applied to soil in one cropping cycle has any effect on plant growth in a relay cropping system, where another crop is planted immediately after harvest of a standing crop. In this study, the carry over effects of two possible soil entry pathways of Fluridone were tested, and Maize (Zea mays L.) was used as test plants because of its relative importance as a crop, and it is well studied and predictable. The study was essentially a bioassay study to determine the effect of soil applied Fluridone on the germination and establishment of maize seedlings, to decipher its possible effect on subsequent crops, using maize (Zea mays L.) as a test crop.
2. Materials and Method
2.1. Experimental Site and Test Soil
- The soil used from this experiment was obtained following a previous experiment using Fluridone as the growth hormone, ABA, inhibitor in yam. The soil for the yam experiment was obtained from a fallow re-growth bush near the Faculty of Agriculture, University of Port Harcourt, Nigeria (4° 54.2'' N and 6° 55.0'' E). The study site is located in the humid tropics and it is characterized by high rainfall lasting from March/April through November, with a short dry season occurring in between. The relative humidity and temperature is high throughout the year.The surface litter was removed and then top 15 cm of the soil collected into large polythene bags and homogenized and amended with poultry manure before putting 5 kg of the soil into plastic pots and planting with non-dormant yam tuber cuttings. In the course of the growth of the yams, 300 ml of 30 µmol Fluridone solution were applied, in three equal splits at 2 days intervals, either directly to the soil in sets of pots, or sprayed on yam leaves. The soil in the pots receiving foliar sprays were screened during spraying to avoid inadvertent application of Fluridone, while another set of pots received no Fluridone (Control).At the end of the yam experiment, the soils were let to dry and then stored for weeks in black polythene bags under ambient conditions in the laboratory prior to this experiment. The soil was slightly acidic (pH 5.6) sandy loam with low clay and moderate organic matter contents.
2.2. Experimental Design and Treatment
- The soils described in Section 2.1 above were measured into small plastic pots (250 g per pots) in three replicates, wetted to field capacity and two maize varieties sowed as test plants (Section 2.3). The experiment was conducted in a screen-house roofed with transparent polythene sheets, with its sides covered sealed with plastic mesh to exclude insects, and lasted for 21 days (from 10th October to 31st October, 2017). The experiment was a 2 x 3 factorial design with 2 maize varieties and 3 Fluridone treatments in three replications. The two maize varieties used were: Sammaz-40 and Oba Super-6, while the Fluridone treatment included: 1) No Fluridone - NF, 2) Previous Soil-Applied - PSA Fluridone, and 3) Previous Foliar-Applied - PFA Fluridone, as described in Section 2.1.
2.3. The Test Plants
- Two varieties of maize were used as test plants: 1) Sammaz 40 and 2) Oba Super 6. Sammaz-40 is an open-pollinated variety of maize jointly released by the Institute of Agricultural Research and Training (IAR&T) of the Ahmadu Bello University (ABU) Zaria, and the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. The yellow pro-vitamin A fortified Sammaz-40 was released in 2013, adapted to the Savannah and capable of yielding 4-5 ton ha-1 [13]. Oba Super 6 is a single cross maize hybrid produced by Premier Seeds Nig Ltd., it is adapted to the moist Guinea Savannah, tolerant to low soil nitrogen and yielding as high as 7-8 ton ha-1 [14].
2.4. Plant Sampling/Data Collection
- Germination tests were carried out on the seedlings of the maize varieties used. For germination tests, the maize seeds were physically evaluated and tested for density by floatation, and 100 seed were selected for each of the variety in three replicates and used to test for germination rates. Based on the results of the germination tests, 12 nos. seeds of Sammaz-40 and 10 nos. seeds of Oba-Super 6 were planted per pot for germination (seedling emergence) tests in the treated soils. The number of seedling emerged each day were counted and recorded.Plant height was measured, using a 1.0 mm graduated rule, from the base of the stem to the tip of the longest leaf (by raising the leaves upwards against the rule). Height measurements were repeated at 7, 9, 14 and 21 days after planting (DAP).Chlorophyll content of the seedling was measured non-destructively, at 9, 18, and 21 DAP, using at LEAF® chlorophyll meter. The atLEAF® chlorophyll meter is a hand-held device and manufactured by FT Green LLC (Washington USA). The equipment, which debuted in 2013, is powerful and easy-to-use tool that measures the chlorophyll content of green plant leaves over an area of 9 mm x 9 mm [15]. The atLEAF® chlorophyll meter works by measuring the transmission of red light (at 660 nm, i.e., wavelength where chlorophyll absorbs light) and also transmission of infrared light (at 940 nm wavelength at which no light is absorbed by chlorophyll).Leaf Area (LA) of maize seedlings we measured at 21 DAP by measuring the length of the each leave (from the tip to the base) and width of the mid-section of the leave. The LA of maize seedlings were estimated following procedures described by [16], using the formula 0.75 (L x W) - where L is the length and W is width of the each leaf. The LA formula was based on the shape of the maize leaves which is rat rectangular with tapered ends [16].
2.5. Statistical Analysis of Data
- The data sets collected in the course of this trial were subjected to statistical analysis of variance (ANOVA) using G.enstat® Computer software.
3. Results and Discussion
3.1. Maize Seed Germination
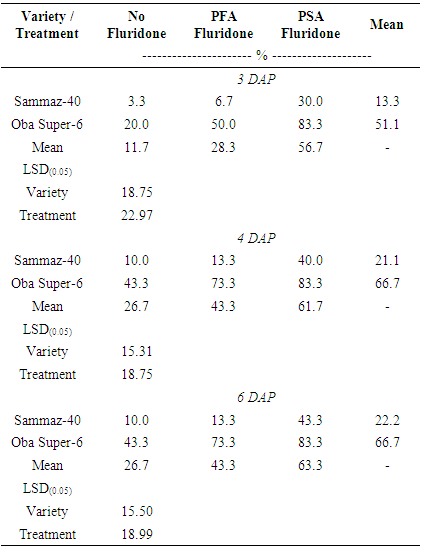
- The results of statistical analysis of germination data showed significant (P < 0.05) differences between the two maize varieties, and between the various soil treatments (Table 1). However, there were no significant interactions (P = 0.05) between the maize varieties and the Fluridone treatments. Within three days after planting, more than half of all the Oba-Super 6 hybrid maize seeds planted had emerged, as compared to only 13% of the Sammaz-40 seeds (Table 1).
|
3.2. Maize Seedling Height
- The results of statistical analysis showed significant (P<0.05) differences among the maize varieties and also the Fluridone treatments, with insignificant (P=0.05) interactions between variety and treatments. Oba-Super 6 grew significantly (P<0.05) than Sammaz 40 within the first 10 days of emergence (Fig. 2). Thereafter, the heights of the two varieties did not differ significantly, implying that the earlier difference in height could be, in part, due to the fact that Oba-Super 6 emerged earlier than Sammaz 40 (Table 1).The effect of Fluridone treatment, however, was not significant in terms of height, in the first two weeks after emergence. By the third week, however, maize grown on the PFA Fluridone treated soil was significantly taller than the PSA Fluridone or No Fluridone Control treatments (Fig. 2).
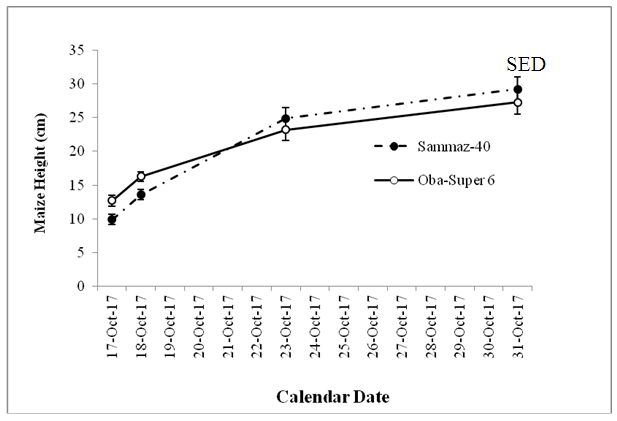 | Figure 1. Effect of maize variety on height |
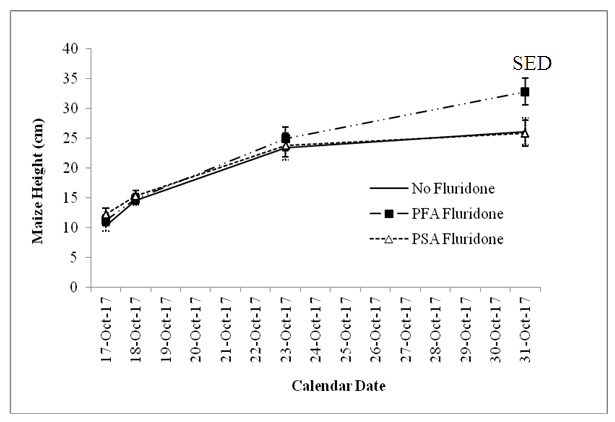 | Figure 2. Effect of soil treated with Fluridone on height |
3.3. Maize Seedling Leaf Chlorophyll Content
- The results of non-destructive chlorophyll measurements are displayed in Fig, 3 and 4. As shown in Fig. 3, Oba-Super 6 had significantly lower (P<0.05) chlorophyll than Sammaz 40. Similarly, the PSA Fluridone treatment had significantly reduced chlorophyll content than either the PFA Fluridone or the Control treatments, while the effects of PFA Fluridone and the treatments did not differ.
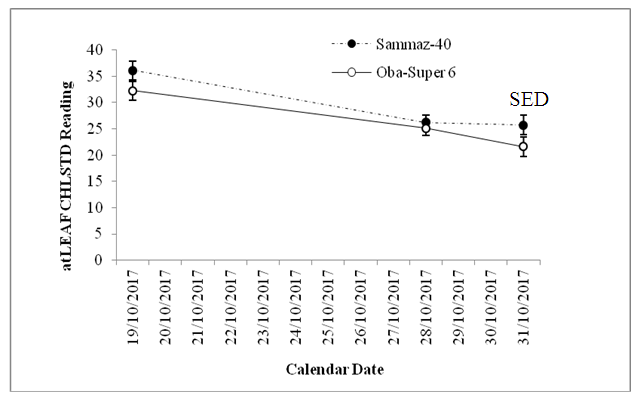 | Figure 3. Effect of variety on chlorophyll contents of maize |
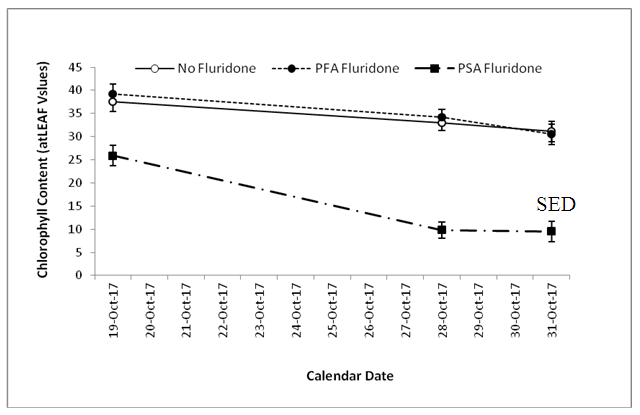 | Figure 4. Effect of Fluridone on Chlorophyll Content |
3.4. Maize Seedling Leaf Area
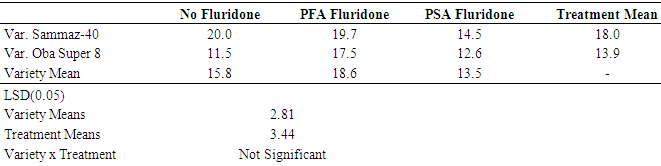
- The results of leaf area measurements for seedling of both Sammaz 40 and Oba Super 6 are shown in Table 2. The leaf area of Sammaz 40 was significantly higher than that of Oba-Super 6. This contrasts data on germination and seedling height), which showed Oba-Super 6 performing better than Sammaz 40. However, a critical observation showed that the differences in germination and emergence rates could be due to inherent varietal differences: Oba-Super 6 matures earlier than Sammaz 40; therefore, it should be expected to germinate and grow faster. During the germination and emergence process, maize seedlings rely on the nutrients stored in the germinating seeds.
|
4. Conclusions
- This study shows that Fluridone improved the germination rates of the maize tested. The direct soil application of Fluridone did not affect the growth of maize, but indirect application did increase maize by 15 DAP. Fluridone applied directly to soil reduced leaf area and chlorophyll contents irrespective of the variety of maize. Notwithstanding that Fluridone improved seed germination; the carryover effects of soil-applied Fluridone can be deleterious to young seedlings of maize as evidenced by the chlorosis of maize leaves and stem. The results of this study trial suggest that direct application of Fluridone to soil in the humid tropics should be avoided as its effects would last beyond the standing crop. Also, it is difficult to tell whether the observed effects of Fluridone on maize could also occur in other crops or not, especially with crops with different growth habits. Therefore, there is need for further investigations to elucidate the residual effects of Fluridone on other crops such as legumes and other broadleaf species. Also, it is worthwhile to investigate whether the effects of soil Fluridone persists across seasons or not.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML