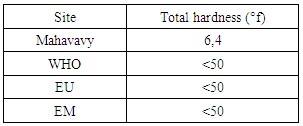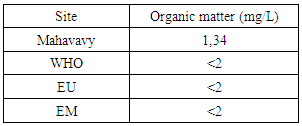Razafitsiferana Théophile, Bruno Razanamparany, Mihasina Rabesiaka, Mahandrimanana Andrianainarivelo
University of Antsiranana, Faculty of Sciences, Chemical Sciences, Mineral Chemistry, Madagascar
Correspondence to: Razafitsiferana Théophile, University of Antsiranana, Faculty of Sciences, Chemical Sciences, Mineral Chemistry, Madagascar.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The Region of DIANA: it is a set of 5 Districts like; Diego I, Diego II; Nosy-Be; AMBANJA and AMBILOBE. My research on the Mahavavy River, in the district of AMBILOBE, is to the south of the chief place of DIANA that is to say of Diego Suarez. The objective of this work is to determine the concentrations of: Physical parameters such as: the temperature is 19.6°C., the turbidity is 3.26 NTU per turbidimeter, the pH is 7.21 by the pH meter, and the conductivity is 53.8 μs/cm measured by conductivity meter, for the three required standards (MS, WHO, EU). For chemical parameters: 50 mg/L mineralization, total hardness 6.5 °f, calcium hardness 2.5 °f, and Alcalimetric titre 0 °f, Calcium 10 mg/L; Magnesium 9.48 mg/L; Carbonates 0 mg/L; Bicarbonates 29.28 mg/L; Organic Matter 1.34 mg/L; Ammonium 0.16 mg/L; Total iron 0.02 mg/L; Chlorides 4.26 mg/L; Sulfates 4.58 mg/L; Nitrites 0.03 mg/L; Nitrates 4.39 mg/L; Sodium 2.76 mg/L, the concentrations found are admissible to the three international standards, despite some deficiencies of measured concentrations such as: Calcium, Magnesium and Chloride, the method used to measure cations such as Sodium, Calcium, Iron and magnesium is spectrometry, atomic absorption with flame. For the microbiological: Coliform bacteria > 2400 NPP/100mL with the method ISO 9308; Escherichia Coli 2 NPP/100mL by the method ISO 9308 NPP/100mL and Intestinal Enterococci 1 NPP/100mL by the method IDX 33/03- 10/13, and the criteria are all null, therefore the elements sought do not meet the required criteria.
Keywords:
Water, Physicochemical and microbiological parameters
Cite this paper: Razafitsiferana Théophile, Bruno Razanamparany, Mihasina Rabesiaka, Mahandrimanana Andrianainarivelo, Analysis of the Physico-Chemical Parameters of the Water of the River of Mahavavy Situated in the District of Ambilobe, in the Region of Diana (DIEGO SUEREZ), Resources and Environment, Vol. 7 No. 6, 2017, pp. 168-175. doi: 10.5923/j.re.20170706.03.
1. Introduction
The district of AMBILOBE is among the 5 districts that make up the Region of DIANA, it is located of 125Km to the South of Diego Suarez. The population of AMBILOBE has about 350 000 women and men and 70% who drink and use the Mahavavy River totally. This for my research is done totally on this River, is that the water used is meets the requirements for drinking required for international standards? So this study is divided into four parts: The 1st bibliographic synthesis, the 2nd measurement results, the 3rd interpretation and discussion of these results, the 4th treatment for microbiological and we will conclude with the conclusion.
2. Bibliographic Synthesis
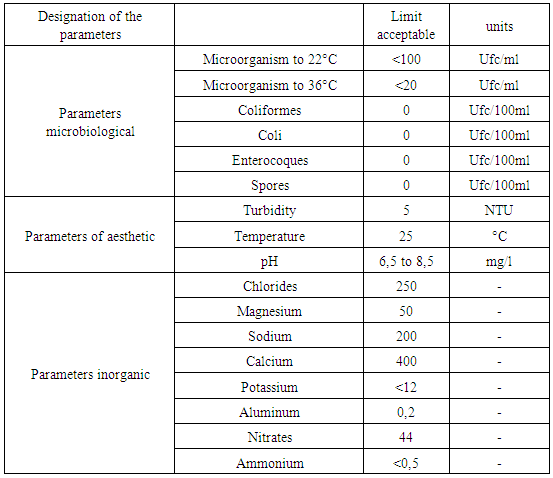
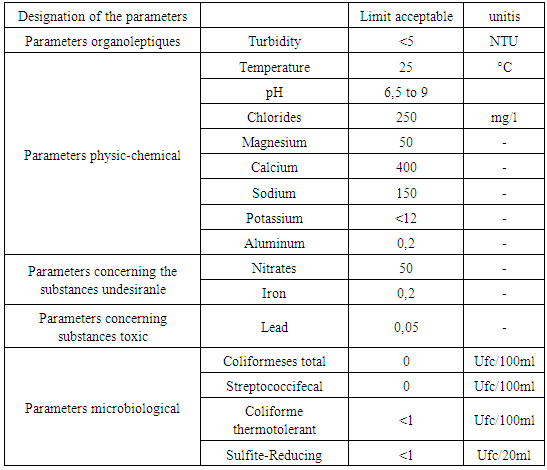
The general formula of water is H2O, there are three possible structures: liquid, solid and gaseous. The dissociation in H + and in OH- represents the chemical operation of the water, the Potential of Hydrogen makes it possible to measure the separation of the two ions. The water contains several organic materials presented in dissolved forms. Water constitutes dissolved oxygen-based gases that represent the chemical composition of water. Cations can exist in water such as: Sodium, potassium, magnesium in various concentrations. Metals also like: Total iron; Lead, Copper represent in trace forms. Pollution these very dangerous in water, existence in water present a microbe. Quality standard Reference is made to the recommendation of the European Union (U E), the World Health Organization (O M S) and the Malagasy State (E M).Norm of quality1 - Recommendation of the WHO 2 - Recommendation of EU 3 - Recommendation of the EM Table 1. Recommendation of the WHO
 |
| |
|
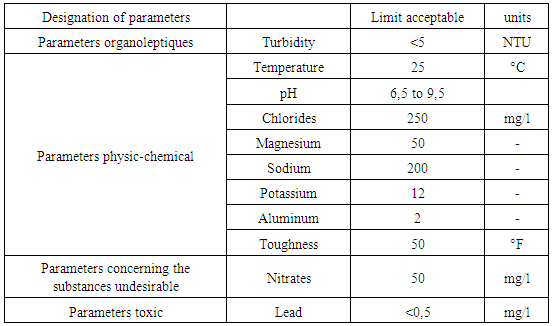
Table 2. Recommendation of the EU
 |
| |
|
Table 3. Recommendation of the EM
 |
| |
|
3. Parameters of Analysis
Dosages of iron to know the concentration of iron in the water is in the form of trace, their existence in water is very important for the calcium and magnesium content for the life of the man. Cations such as: Aluminum, Lead, Copper are lord metals their existences in drinking water is not accepted to international standards. Chlorides is a major ion contained in natural water, their presence is very important in drinking water. The concentrations of Calcium and Magnesium in drinking water are very high on all three international standards. Ammonium, Nitrate and Nitrite, their existences in water represent pollution. Title Alkalimetry (T A), Title Alkalimetry Complete (T A C), Carbonates and Bicarbonates: to know the concentration of OH- ion in water, it is the basic salt content. Organic materials such as BOD and COD can be used to estimate the amount of organic matter in the water. To know the quantity of salt dissolved in water, conductivity can be measured. To know that the water is acidic or basic one can measure the pH. To know the transparency of water, one measure the turbidity is that the water is clear.
4. Results of Measures
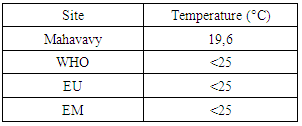
I- Physical parameters1- TemperatureTable 4. Represents the measurement of the temperature
 |
| |
|
 | Figure 1. Temperature measurement |
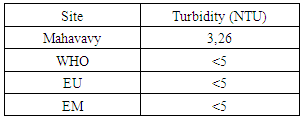
The value of the temperature found is 19.6°C, is eligible for the three standards required for drinking water [4].2 – TurbidityTable 5. Represents the measurement of the Turbidity
 |
| |
|
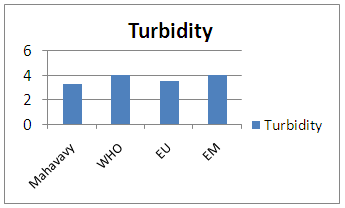 | Figure 2. Turbidity measurement |
The measurement result for turbidity is 3.26 NTU therefore lower than 5NTU it is acceptable for drinking water [1, 2].3 – pHTable 6. Represents the measurement of the pH
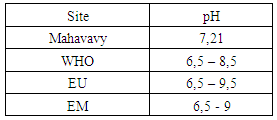 |
| |
|
 | Figure 3. pH measurement |
The pH value found is 7.21 therefore between 6.5 and 9 on average for standards of potability, water is good [4, 5].4 – ConductivityTable 7. Represents the measurement of the conductivity
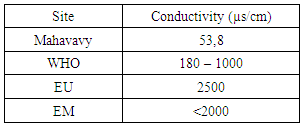 |
| |
|
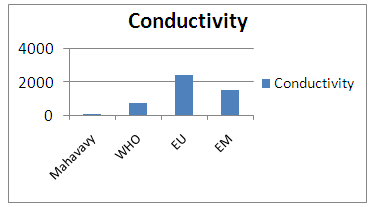 | Figure 4. Conductivity measurement |
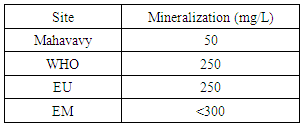
The conductivity measurement result is 53.8 μs/cm, between 180 - 1000 μs/cm, and is therefore acceptable for drinking water.II- CHEMICAL PARAMETERS1 – MineralizationTable 8. Measurement results of Mineralization concentration
 |
| |
|
 | Figure 5. Mineralization measurement |
There is a relationship between mineralization and conductivity: if the conductivity is lower than 100 μs/cm as already found here 53,8 μs/cm, the mineralization is very low; and for the mineralization to be large it is necessary that the conductivity between 666 - 1000 μs/cm [3].2 - Total hardnessTable 9. Represents the measurement of the Total hardness
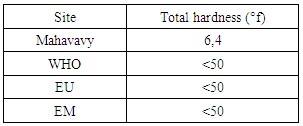 |
| |
|
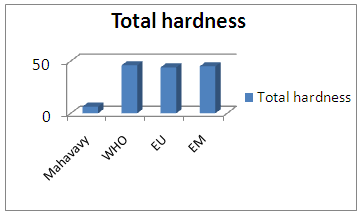 | Figure 6. Total hardness measurement |
The value of the total hardness found is less than 50°, so the water is not known, but the low hardness can cause corrosion problems [7].3 - Alkalimetric Title (T A)Table 10. Represents the measurement of the Title Alcalimetric
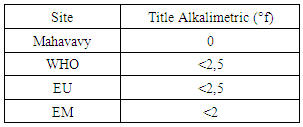 |
| |
|
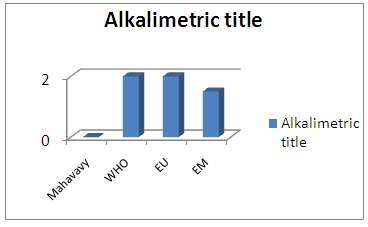 | Figure 7. Alkalimetric title measurement |
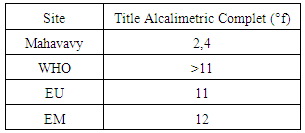
The TA value does not exist in this sample [6].4 - Full Alkalimetric Title (T A C)Table 11. Represents the measurement of the Full Alkalimetric Title
 |
| |
|
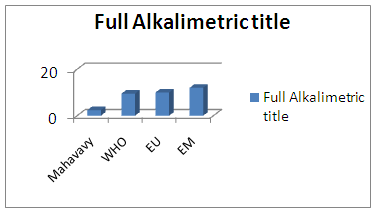 | Figure 8. Full Alkalimetric title measurement |
The TA is equal to zero and TAC = HCO-3, so the quality of the water is on average [6].5 – CalciumTable 12. Represents the measurement of the calcium
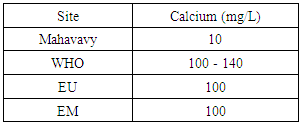 |
| |
|
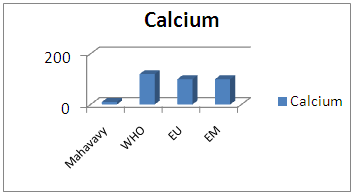 | Figure 9. Calcium measurement |
The calcium concentration is 10 mg/L, very low 100 mg/L, so this sample is low in calcium content [10, 15].6 – MagnesiumTable 13. Represents the measurement of the magnésium
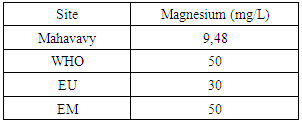 |
| |
|
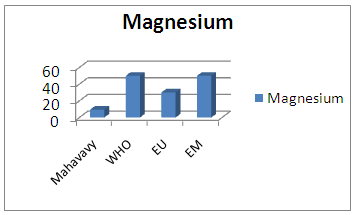 | Figure 10. Magnesium measurement |
The concentration of magnesium is 9.48 mg/L, lower than 30 mg/L, the magnesium content is low [10, 15].7 – BicarbonatesTable 14. Measurement results of Bicarbonates concentration
 |
| |
|
 | Figure 11. Bicarbonates measurement |
The surface water does not contain more than 10 mg/L of Bicarbonate, but in this sample the concentration found is 29.28 mg/L, so the water is not good.8 - Organic matterTable 15. Measurement results of Organic matter concentration
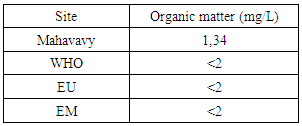 |
| |
|
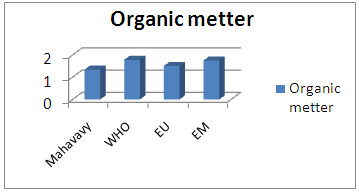 | Figure 12. Organic matter measurement |
The concentration of organic matter is 1.34 mg/L, therefore less than 2 mg/L for the values required by international standards [3].9 – AmmoniumTable 16. Measurement results of Ammonium concentration
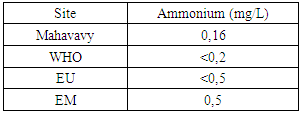 |
| |
|
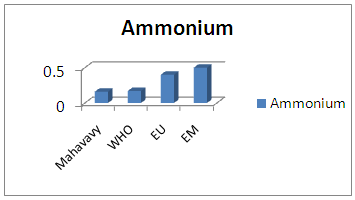 | Figure 13. Ammonium measurement |
The concentration of ammonium is 0.16 mg/L, lower than the values required for drinking water standards. [9]10 - Total IronTable 17. Measurement results of Total Iron concentration
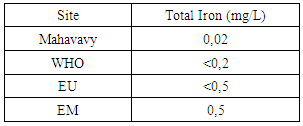 |
| |
|
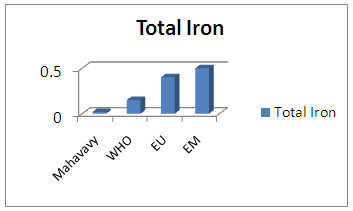 | Figure 14. Total Iron measurement |
The concentration of total iron is normal compared to the concentrations required for drinking water [14].11 – ChloridesTable 18. Measurement results of Chlorides concentration
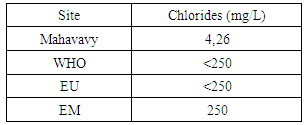 |
| |
|
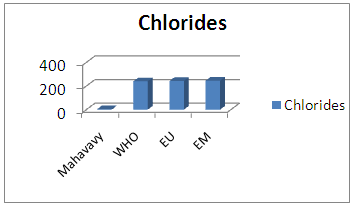 | Figure 15. Chlorides measurement |
The concentration of chloride is very low compared to the values required for drinking water [12].12 – SulphatesTable 19. Measurement results of Sulphates concentration
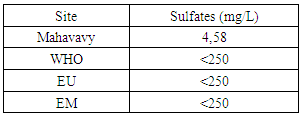 |
| |
|
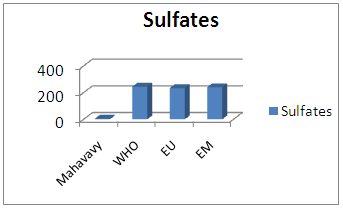 | Figure 16. Sulphates measurement |
Sulphate concentration is also very low compared to international standards for drinking water.13 – NitritesTable 20. Measurement results of Nitrites concentration
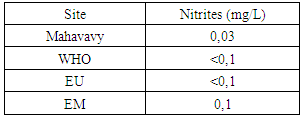 |
| |
|
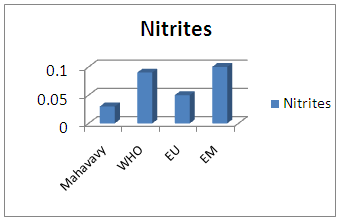 | Figure 17. Nitrites measurement |
The nitrite concentration is acceptable for all three drinking water standards.14 -NitratesTable 21. Measurement results of Nitrates concentration
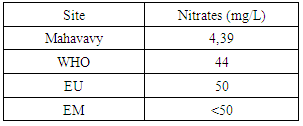 |
| |
|
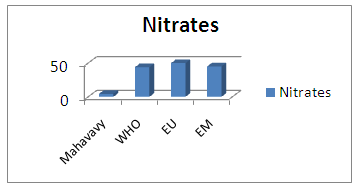 | Figure 18. Nitrates measurement |
The nitrate concentration is very low compared to the three international standards [16].15 – SodiumTable 22. Measurement results of Sodium concentration
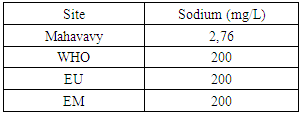 |
| |
|
 | Figure 19. Sodium measurement |
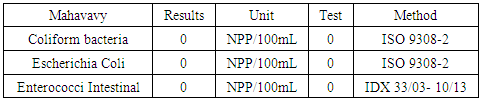
The sodium concentration is very low compared to the three standards required for drinking water.III- MICROBIOLOGICAL ANALYSISTable 23. Measurement results of the microbiological
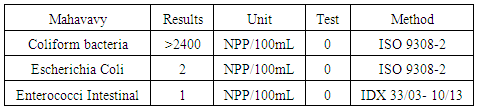 |
| |
|
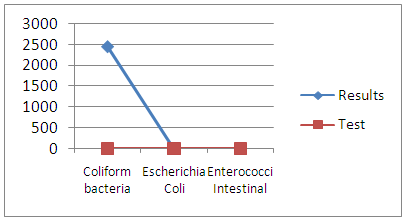 | Figure 20. Microbiological measurement |
The elements sought do not meet the criteria for drinking water.
5. Results Interpretation
- Physical parameters:The temperature is 19.6°C, the turbidity is 3.26 NTU, the pH is 7.21 and the conductivity is 53.8 μs/cm, the values found are below the values required by the standards of drinking water, so the Mahavavy River water at the physical parameters are good.Chemical parameters- Organic matter is equal to 1.34 mg/L, is normal compared to the three standards required, if BOD5 < 3 mg/L is taken as this sample 1.34 mg/L, the water quality is very good.- The total hardness and calcium hardness are: 6, 9 and 2, 4 °f makes it possible to speak that the water of the sample that we are going to study is not hard.- The alkalimetric titre and the complete alkalimetric titre are: 0 and 2.4 °f these values show that the carbonate content in the water is low.- The concentration of Calcium and Magnesium are: 10 mg/L and 9.48 mg/L are admissible for the values required by the standards of potability, despite their deficiencies of the concentration found.- The chloride and sulphate are: 4.26 mg/L and 4.58 mg/L the concentrations found are less than 250 mg/L required for drinking water.- Ammonium is equal 0.16 mg/L, less than 0.5 mg/L the required value, so the water is not polluted.- The carbonate and the bicarbonate are 0 and 29, 28 mg/L, the water of the sample that we will study are lack of carbonate.- The concentration of sodium is 2.76 mg/L; it is very low by international standards.Microbiological analysisThe river water of the mahavavy is microbial, it is necessary to treat that before use, so I propose a method of disinfection by Chlorine.Treatment of water by chlorinationChlorine is the most widely used reagent for disinfection of water. It is endowed with a very important remanent oxidizing power, favorable to the destruction of the organic matter.Table 24. Measurement results are available immediately after disinfection
 |
| |
|
 | Figure 21. Measurement results are available immediately after disinfection |
A minimum contact time of 30 minutes after which the residual chlorine dose must still be 0.1 mg/L at 0, 2mg/L.This water is taken to make an analysis because only the bacteriological examinations ensure a perfect control of the disinfection. Measurement results are available immediately after disinfection [13].The values found are perfectly suitable for drinking water.
6. Conclusions
The physical parameters perfectly meet the requirements for drinking water.The chemical parameters correspond to 90% of the conditions required for the water to be potable, damage some deficiencies of the concentrations found in calcium, magnesium and sodium.Bacteriological, mahavavy river water is microbial; it must be disinfected before being used. So the proposal is already given the disinfection of water by a Chlorination method.
References
| [1] | J. RODIER, M FAVRE-DUBOZ (1975). Remarques sur les mesures de turbidité des eaux. L’eau. 12, p. 263. |
| [2] | G. NOISETTE (1959). Relation entre mesure de turbidité et matières en suspension non décantables C.B.E.D.E. Bull. trimestriel, III, (45). P. 139. |
| [3] | JJ. MCKEOWN et al. (1967). Comparative studies of dissolved oxygen analysis methods.J. Water Pollut. Control Fed. 39; 1323. |
| [4] | W.F. LANGELIER (1964). Effect of temperature on the pH natural waters. J.A.W.W.A. 38. P. 179. |
| [5] | R.G.BATES (1978). Concept and determination of pH. In I.M. Kolthoff. |
| [6] | T.E. LARSON and L.M. HENLEY (1955). Determination of low alkalinity or acidity in Water. Anal. Chem. 27; 851. |
| [7] | J. RODIER. CH. GRAUDE (1952). Détermination de la dureté dans les eaux par la method au complexon III.Bull.institut d’Hygiène du Maroc. N.S XII ? (3-4).p.275. |
| [8] | G.WAUER et al. (2004). Analysis of toxic Aluminium Species in Natural Waters, Michrocimica Acta, 146; 149. |
| [9] | C. MOLINS-LEGUA et al. (2006). A guide for selecting the most appropriate method for ammonium determination in water analysis, Trends in Analytical Chemistry, 25; 282. |
| [10] | H.KATZ.R. NAVONE (1964). Method for simultaneous determination of calcium and magnesium, J.A.W.W.A, 1. p.56. |
| [11] | F.E.CLARKE (1950). Determination of chloride in water. An.Chem.22.p.553-1458. |
| [12] | F.SAGARE et al. (1992). Determination of chloride ion concentration in natural and waste waters by flow-injection analysis with a silver chloranilate column, Analytical Chimica Acta. 270:787. |
| [13] | R. BUYDENS et R. MUYLLE (1952). Dosage du fer dans les eaux. C.B.E.D.E. IV, (18). p. 241. |
| [14] | F. CANETTE et al. (1987). Determination of analytical parameters in drinking water by flow injection analysis. Part 2. Simulataneous determination of calcium and magnesium, Analyst, 112; 267. |
| [15] | S.C. PAL et J.P.RILEY (1996). Determination of Nitrate in the presence of Nitrite in natural waters by flow injection analysis with a non-quantitative on-line cadmnium redactor, international Journal of Environnemental Analytical Chemistry, 57: 263. |
























 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML