-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2017; 7(5): 138-144
doi:10.5923/j.re.20170705.04

Cellular Uptake and Metabolism of High Molecular Weight Polycyclic Aromatic Hydrocarbons by the White-rot Fungus Phanerochaete chrysosporium
Nomathemba Loice Chigu1, 2, Hirofumi Ichinose1
1Faculty of Agriculture, Kyushu University, Fukuoka, Japan
2School of Agricultural Sciences and Technology, Biotechnology Department, Chinhoyi University of Technology, Chinhoyi, Zimbabwe
Correspondence to: Nomathemba Loice Chigu, Faculty of Agriculture, Kyushu University, Fukuoka, Japan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Polycyclic aromatic hydrocarbons (PAHs) are lipophilic molecules that generally partition into lipid rich tissues in living organisms where they can be actively metabolized by cell-associated enzymes. In this study, cellular uptake and accumulation of triphenylene, benzo(a)pyrene, and coronene in lipid vesicles of the fungus Phanerochaete chrysosporium ATCC 34541 was investigated. Numerous bodies that stained with the lipid specific dye Sudan III were observed in fungal hyphae of P. chrysosporium grown with the high molecular weight (HMW) PAHs, implying that they could be taken in by the fungus and stored in lipid vesicles. Furthermore, the subsequent metabolism of the HMW PAHs by the fungus was investigated. This fungus degraded benzo(a)pyrene significantly and showed poor degradation activities for triphenylene and coronene suggesting that fungal intracellular accumulation could not essentially accompany degradation, therefore evaluating transport of compounds in cells may help to discern lack of substrate metabolism due to enzyme specificity or insufficient enzyme contact.
Keywords: High molecular weight polycyclic aromatic hydrocarbons, Cell accumulation, Intracellular enzymes, Lipid vesicles, Ionization potential
Cite this paper: Nomathemba Loice Chigu, Hirofumi Ichinose, Cellular Uptake and Metabolism of High Molecular Weight Polycyclic Aromatic Hydrocarbons by the White-rot Fungus Phanerochaete chrysosporium, Resources and Environment, Vol. 7 No. 5, 2017, pp. 138-144. doi: 10.5923/j.re.20170705.04.
Article Outline
1. Introduction
- The white-rot fungus Phanerochaete chrysosporium shown in figure 1 has been shown to be an ubiquitous degrader of an extensive array of xenobiotics compounds ([4, 5, 7, 24, 32, 39, 56, 61]) including polycyclic aromatic hydrocarbons (PAHs).
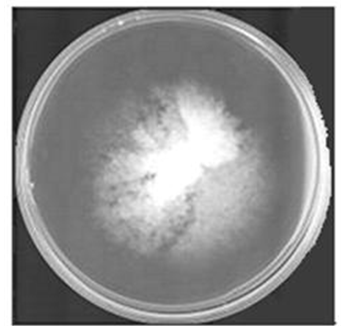 | Figure 1. P. chrysosporium maintained on PDA media |
|
2. Materials and Methods
2.1. Chemicals
- Anthracene, triphenylene, benzo(a)pyrene and coronene were purchased from Wako pure chemicals (Osaka, Japan). Sudan III was supplied from Kasayama. All other chemicals were reagent grade. Deionized water was obtained with a Milli Q System (Millipore).
2.2. Metabolism of PAHs by the Fungus P. chrysosporium
- P. chrysosporium (ATCC 34541) was grown from conidia inocula at 37°C in a stationary culture under air (10 mL of medium in a 100-mL Erlenmeyer flask). The medium used in this study was as previously described [31] with 1% glucose and 1.2 mM ammonium tartrate at pH 4.5.After a 4-day pre-incubation, substrate was added to the cultures to become final concentration of 25 µM. Coronene in dimethyl formamide (2.5 mM), triphenylene and benzo(a)pyrene in acetonitrile (2.5 mM) were utilized substrates-stock solution. Anthracene (2.5 mM) was used for co-metabolic studies with coronene. NaN3 (1 M) treated cultures were used as controls in the biodegradation studies. To detect abiotic degradation of substrates, blanks without fungi were prepared and processed analogously. Cultures were incubated at 37°C without agitation under 100% oxygen for periods ranging from 20-30 days. Periodically harvested cultures and controls were homogenized in triplicates with 20 ml acetonitrile, centrifuged at 25,000 x g for 10 min at 4°C to separate the mycelium from the aqueous fraction and filtered with a membrane (0.45 µm). The aqueous fraction obtained was analyzed by HPLC.
2.3. High Performance Liquid Chromatography Analysis
- HPLC analysis of HMW PAH metabolism was done with a Shimadzu STR ODS-II column (4.6 by 150 mm I.D.) Detection of triphenylene and benzo(a)pyrene metabolites was achieved at 257 and 254 nm respectively with a linear gradient from 20% acetonitrile in water, isocratic for 5 mins, to a 100% acetonitrile (21-31 min) at a flow rate of 1.0 ml/min. Coronene samples were detected at 304 nm with a linear gradient from 50% acetonitrile in water, isocratic for 5 mins, to a 100% acetonitrile (21-31 min) at a flow rate of 1.0 ml/min.
2.4. Fluorescence Observations
- Cultures were grown at 37°C without agitation under 100% oxygen for five days after substrate addition. Fungal samples were collected from liquid cultures containing no PAH (control sample) or one of the different PAHs used. Observations were conducted with a fluorescent microscope (Leica). PAHs fluorescence wavelengths range from 210 -380 nm.
2.5. Sudan III Staining Protocol
- Stock solution of Sudan III (0.2% w/v) was prepared using 70% (v/v) ethanol and warmed in a water bath. The solution was then cooled down to room temperature, filtered and kept in dark at room temperature. Fungal cells were washed thrice with distilled water, and twice with acetone to remove PAH residues. The cells were immersed in the working solution of Sudan III in the dark for 20 min. Hereafter sections were washed with 3 exchanges of deionized water for 30s each. Cells were mounted for observation in an aqueous medium and or glycerin [21].
3. Results and Discussion
3.1. Uptake of Polycyclic Aromatic Hydrocarbons by P. chrysosporium
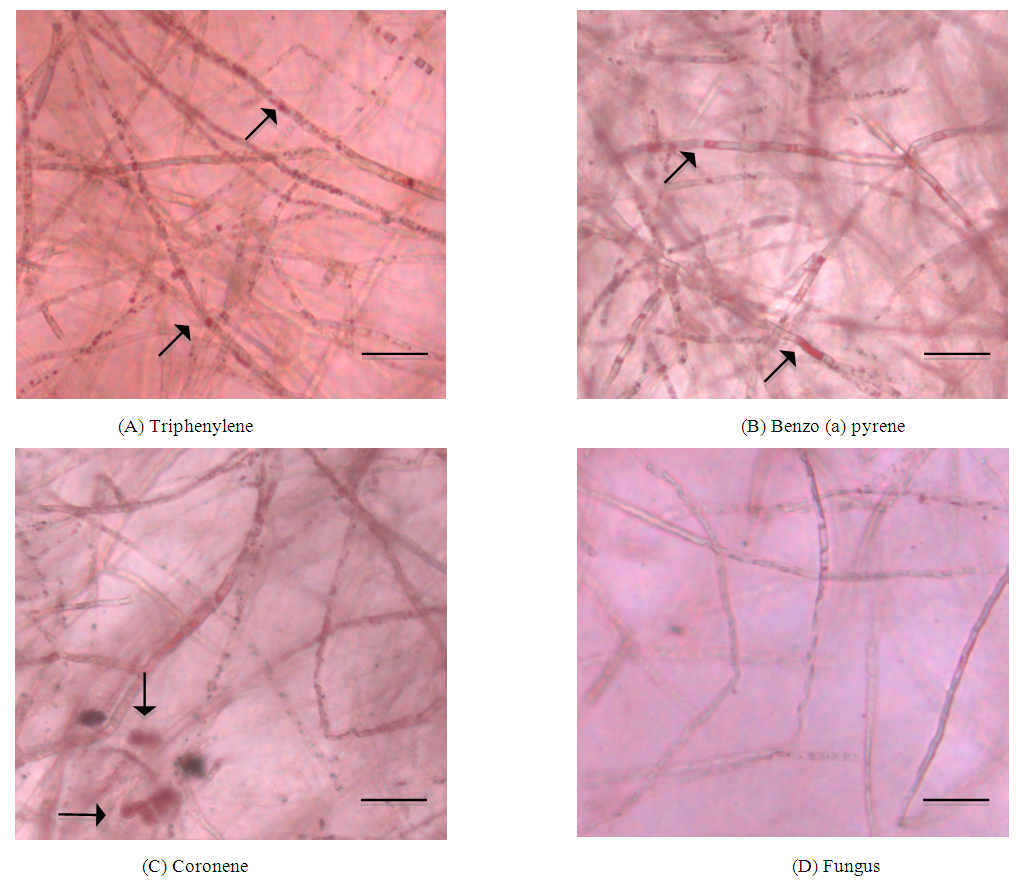
- PAHs are lipophilic molecules that are easily incorporated into lipid vesicles for transport and storage ([43, 54]). PAH containing elements in these lipid vesicles can be visualized with Sudan III and other dyes to stain lipid droplets for fluorescence microscopy. In this study, three recalcitrant HMW PAHs (Figure 2) were separately added to cultures of P. chrysosporium after a 4-day pre-incubation. Their uptake by the fungus during its cultivation was investigated using fluorescence microscopy. The results obtained were compared to those analogously done in the absence of PAHs.
 | Figure 2. Chemical structures of HMW PAHs used in this study |
3.2. Metabolism of HMW PAHs by the White-Rot Fungus P. chrysosporium
- In , [55] demonstrated that fluorescent PAHs concentrate in the lipid droplets of fungi, which sequester these noxious compounds and perhaps metabolize them to less toxic derivatives. After evaluating the uptake of HMW PAHs, a further study was conducted to evaluate the potential ability of fungal degradation of these high molecular weight PAHs (shown in figure 2). The fungus P. chrysosporium degraded Benzo (a) pyrene significantly the results of which have been published [15]. In contrast, the fungus P. chrysosporium showed poor degradation of both triphenylene and coronene (figure 4). Cometabolism of coronene with anthracene, a relatively simple PAH can have multimechanistic effects on coronene metabolism, such as inhibition or induction [59]. In this study, the presence of anthracene in fungal cultures slightly improved the degradation of coronene.
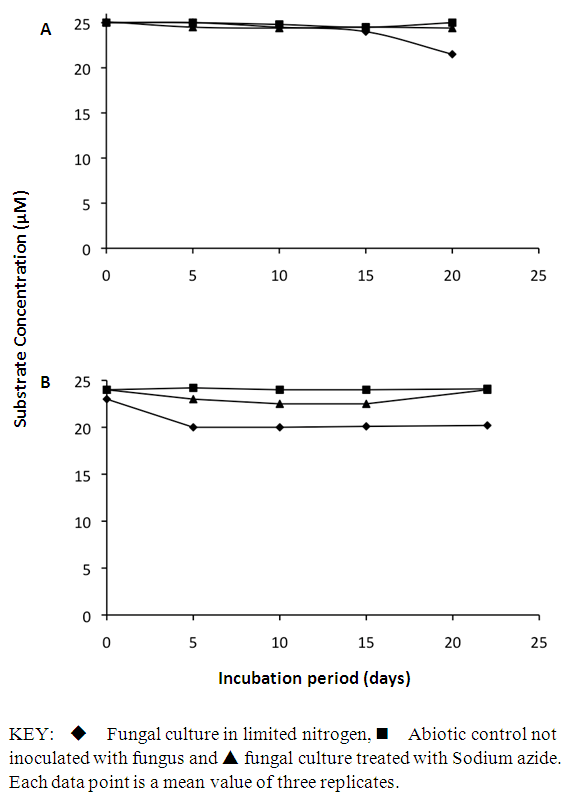 | Figure 4. Time course of (A) Triphenylene and (B) Coronene metabolism by the white rot fungus P. chrysosporium ATCC 34541 |
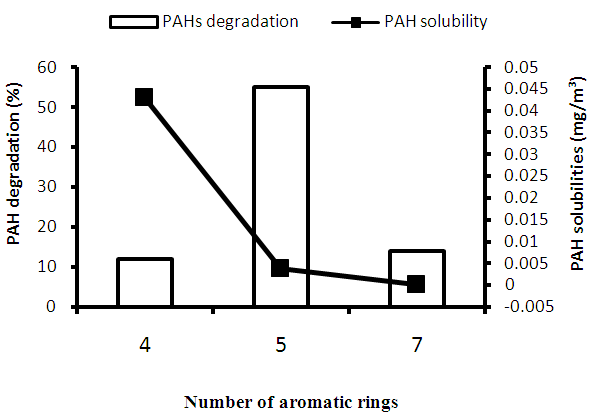 | Figure 5. Correlation between the number of aromatic rings of PAHs, their % degradation and solubilities. 4, Triphenylene; 5, Benzo (a) pyrene; 7, Coronene |
4. Conclusions
- This study is one of the few concerned with the correlation between substrate uptake and metabolism. P. chrysosporium cells grown in the presence of triphenylene, benzo (a) pyrene, and coronene accumulated these PAHs and showed small intensely stained rose-red inclusions that were not observed in fungal cell hyphae lacking the PAHs. This fungus degraded Benzo (a) pyrene significantly but showed poor degradation of Triphenylene and Coronene. Therefore, evaluating transport of these recalcitrant HMW PAH compounds in the cells of P. chrysosporium help to discern lack of attack due to enzyme specificity or due to insufficient enzyme contact. This effort is of both a practical and theoretical interest. Effort to engineer modified enzymes or pathways ([14, 29]) for degradation of recalcitrant compounds would be wasted if the recalcitrance were essentially due to a transport problem.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML