Razafitsiferana Théophile, Bruno Razanamparany, Mihasina Rabesiaka, Mandrimanana Andrianainarivelo
Universite Antsiranana Mention Chimie Minerale, Madagascar
Correspondence to: Razafitsiferana Théophile, Universite Antsiranana Mention Chimie Minerale, Madagascar.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The Nosy-Be island has 12 large sacred lakes, the urban commune of Nosy-Be is a set of five (5) arrondissements, my search is done in the 5th Arrondissement named BEMAGNONDROBE. The first objective of this work; Determining the concentrations of physico-chemical, microbiological parameters and the quality control of these parameters in relation to international standards. The second objective, to satisfy the populations of BEMAGNONDRO BE and also the population drinking water safely. The method used for metals is mass spectrometry. The method for pH: pH-meter, temperature: thermometer, turbidity: turbidimeter, Conductivity: conductivity meter. The results of the physical parameters obtained correspond to 95% of the standards of potability, the chemical parameters almost admitted to the standards by WHO and the EU and while the microbiological correspond exactly to 100% for the norms of the international potabilities.
Keywords:
Water, Physico-chemical, Microbiological and quality control parameters
Cite this paper: Razafitsiferana Théophile, Bruno Razanamparany, Mihasina Rabesiaka, Mandrimanana Andrianainarivelo, Analysis of Physico-Chemical Parameters and Quality Control of Water in Anjavibe Lake in the Bemagnondrobe Borough Situated in Nosy-Be District, Resources and Environment, Vol. 7 No. 5, 2017, pp. 124-130. doi: 10.5923/j.re.20170705.02.
1. Introduction
Nosy-be is an exceptional island because of its geology and its relief, that is to say in the island of Nosy-Be, there are 12 great sacred lakes.The island of Nosy-Be is divided into five (5) arrondissements, so the research is carried out for the borough of BEMEGNONDROBE. The number of inhabitants in this district is given by the following table: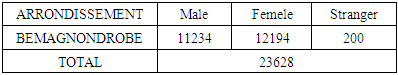 Among the 12 sacred lakes we call lake ANJAVIBE, this lake is located in the borough of BEMAGNONDROBE. It is located in the north of the island. The population in this borough uses this lake as drinking water. So this for cella that we draw from my research aims to analyze the physicochemical parameters and the quality control of this lake called ANJAVIBE to make known the situation of this lake is what drinkable or not.
Among the 12 sacred lakes we call lake ANJAVIBE, this lake is located in the borough of BEMAGNONDROBE. It is located in the north of the island. The population in this borough uses this lake as drinking water. So this for cella that we draw from my research aims to analyze the physicochemical parameters and the quality control of this lake called ANJAVIBE to make known the situation of this lake is what drinkable or not.
2. The Characteristics of Lake ANJAVIBE
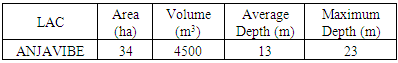 The study of these parameters is different from other research,This study consists of four parts, beginning with the bibliographic synthesis and then the results of measurement for the physico-chemical and microbiological analyzes, followed by the interpretation and the discussion of this result, and ends with the conclusion.
The study of these parameters is different from other research,This study consists of four parts, beginning with the bibliographic synthesis and then the results of measurement for the physico-chemical and microbiological analyzes, followed by the interpretation and the discussion of this result, and ends with the conclusion.
3. Bibliographic Synthesis
Water has the liquid, solid and gaseous structure, the general formula is H2O.The chemical operation of water: is the dissociation in H + and in OH- ion, the separation of the two ions is measured with the Potential of Hydrogen.The chemical composition of the water: consists of dissolved gases of base to oxygen.The water contains several organic materials: in various concentrations, it also contains the organic materials presented in dissolved forms.
4. Norm of Quality
1 - Recommendation of the WHO 2 - Recommendation of EU 3 - Recommendation of the EM Recommendation of the WHO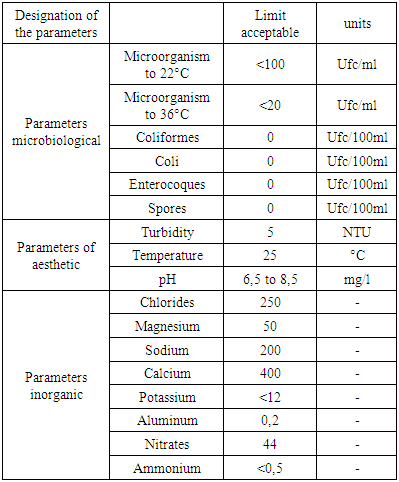 Recommendation of the EU
Recommendation of the EU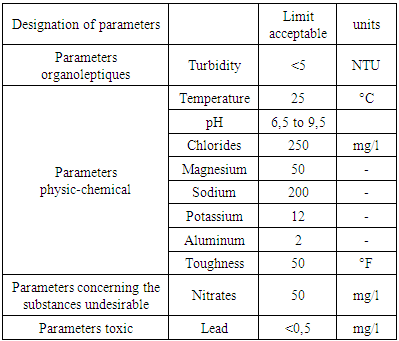 Recommendation of the EM
Recommendation of the EM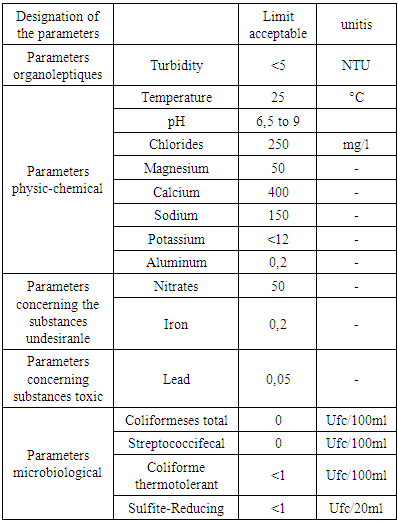 Metals: may exist in water form trace.Pollutions: these very dangerous in water it presents in microbe.Quality standardThe standard of qualities is referred to by the European Union (U.E) recommendation and the World Health Organization (WHO).
Metals: may exist in water form trace.Pollutions: these very dangerous in water it presents in microbe.Quality standardThe standard of qualities is referred to by the European Union (U.E) recommendation and the World Health Organization (WHO).
5. Analysis Parameters
- Turbidity: it is the transparency of water.- pH: to know the water is acid, base and neutral, its depends on the variation of this pH.- Conductivity: allows appreciating the quality of salt dissolved in the water.- Organic matter: allows estimating the quality of organic matter in water, BOD and COD.- Salinity: this is the measure of salt concentration in water.- Alkalimetric title: this is the basic salt content, ie to know the concentration of OH- ion in water.- Determination of nitrate content: Determination of nitrate concentration in water.- Total hardness: Determines the calcium and magnesium content in the water.- Dosages of iron: to know the concentration of iron in water, it is in the form of trace.- Ammonium: the ammonium ion indicates an existence of pollution in water.- Sodium: is responsible for the hydro-electrolyte balance.- Potassium: plays a role as a calcium in human life.- Calcium and magnesium: concentrations are very high in drinking water.- Aluminum, Lead and Copper: are lord metals, their existences in drinking water do not accept for international standards.- Determination of chloride: it is a major ion contained in natural water.
6. Results of Measures
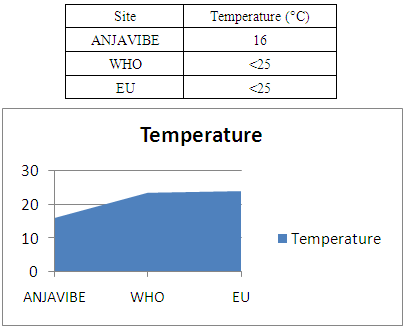
I / -Parameters1- TemperatureThe temperature is in the potability frame, so it is acceptable
 |
| |
|
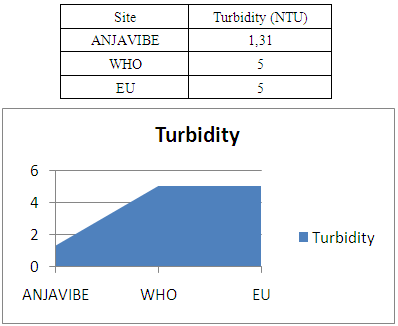
2- TurbidityThe turbidity is equal to 1.31 NTU thus acceptable for the international standard
 |
| |
|
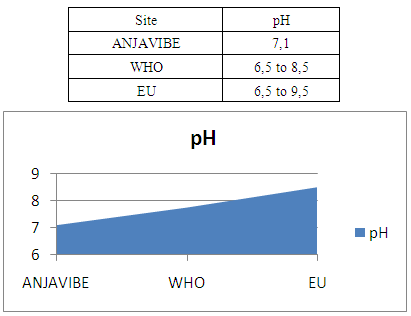
3- The pHThe pH is equal to 7.1 therefore it is acceptable for the quality standard for potability
 |
| |
|
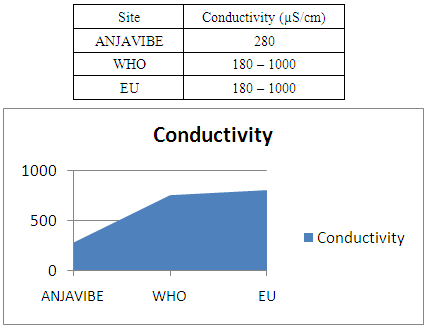
4- ConductivityThe conductivity is 280μs / cm acceptable for the potability standard
 |
| |
|
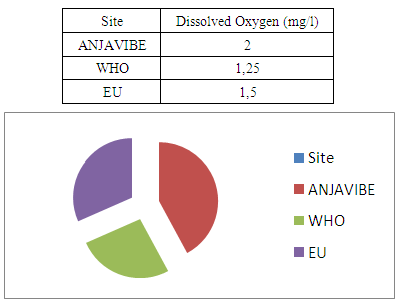
II- chemical parameters1- Organic materialsOxygen dissolved the value found is greater than 2mg / l therefore outside of potability standard
 |
| |
|
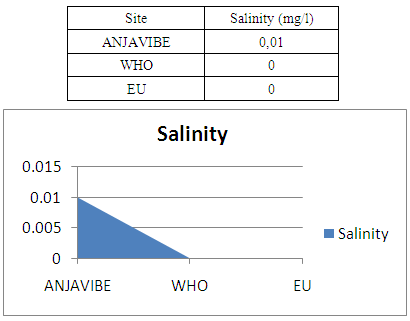
2- SalinitySalinity is zero and therefore normal
 |
| |
|
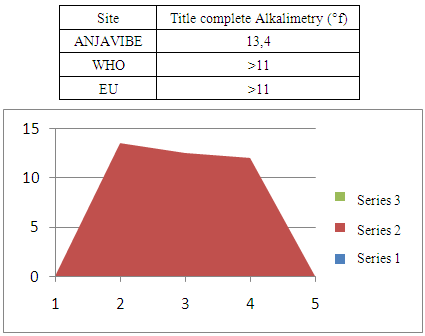
3- Title AlkalimetryThe TAC 13.4 ° f almost normal for the potability of water
 |
| |
|
4- Nitrate assaysNormal nitrate is equal 0.9mg / l water is clear
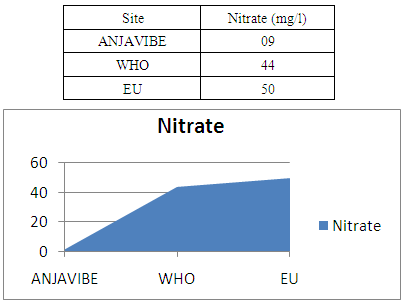 |
| |
|
5- The total hardnessHardness 28mg / l water is not so so normal
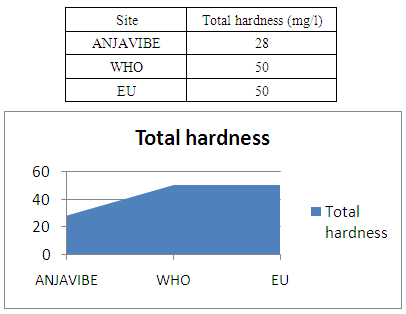 |
| |
|
6- Determination of ironIron 0 mg / l therefore no trace for water
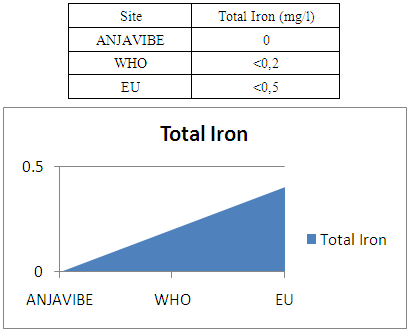 |
| |
|
7- AmmoniumAmmonium 0.01mg / l therefore no pollution water
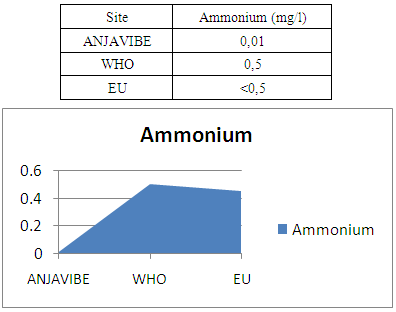 |
| |
|
8- SodiumSodium 4.5mg / l very low so the water lacks a lot of sodium
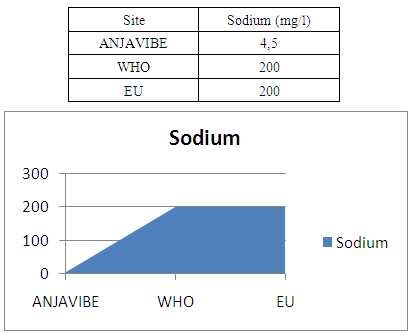 |
| |
|
9- PotassiumPotassium 13mg / l higher normal value
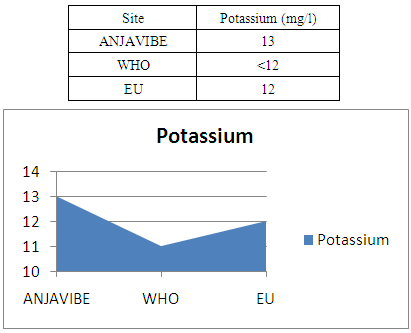 |
| |
|
10- CalciumCalcium 35mg / l, the standard is 400mg / l so the water low in calcium
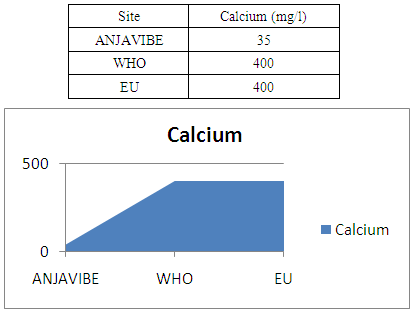 |
| |
|
11- MagnesiumMagnesium is 47.5mg / l value is normal for drinking water
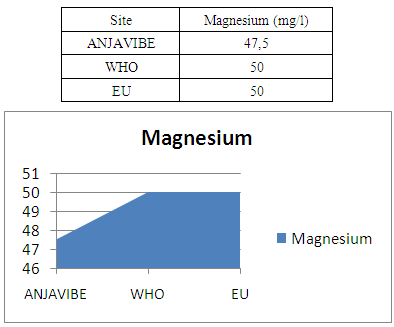 |
| |
|
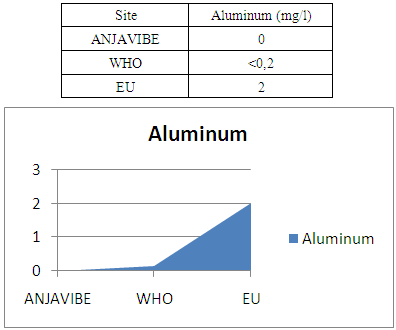
12- AluminumAluminum 0mg / l this water no aluminum
 |
| |
|
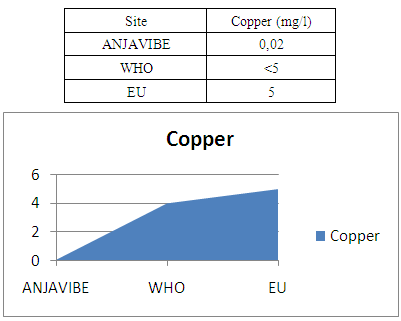
13- CopperThe copper value is 0.2mg / l in trace form
 |
| |
|
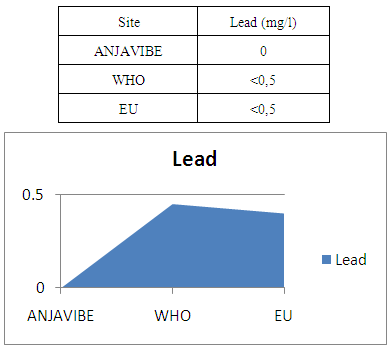
14 - LeadLead 0mg / l water no danger
 |
| |
|
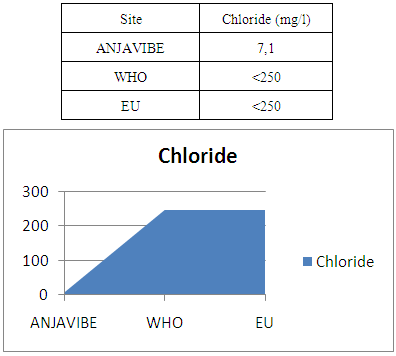
15- Determination of chlorideChlorides 7.1mg / l very low concentration found by international standards
 |
| |
|
7. Bacteriological Analyzes
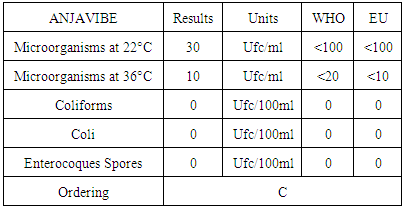
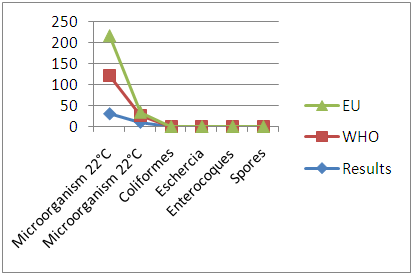
8. Quality Control
In quality control, values on physical parameters, chemical parameters and bacteriological parameters are compared with international standards in order to conclude the potability of water.Physical parametersThe temperature 16°C, turbidity 1.31 NTU, pH 7.1 and conductivity 280 μs/cm [1, 4] are admissible to international standards.The organic matter such as dissolved oxygen is equal to 2 mg / l, this value is outside for the standard by WHO and EU because the required value is <2mg / l. [5]Chemical parametersIn general, the results obtained are admissible to the values required by the potability of the water, despite some inadequacies of concentrations such as calcium is 34.5 mg / l, [5-8].Required value is 400mg / l and the chloride is 7.1 mg / l, the required value is 250mg / l. [9]Bacteriological analysisThe water of the lake called ANJAVIBE that we will study is not microbial [3].
9. Conclusions
The physical parameters of ANJAVIBE lake exactly meet the WHO and EU standards, chemical parameters meet almost 90% of the requirements for water potability and 100% allowable for microbiological conditions.Therefore the water of Lake ANJAVIBE used by the population of the 5th Arrondissement named BEMAMGNOBE in the District of Nosy-be is drinkable.
References
| [1] | W.F. Langelier (1964). Effect of temperature on the pH natural Waters J.A.W.W.A.38, p.179. |
| [2] | C.I. Luke, K.C. Braun (1952). Photometric determination of aluminium. |
| [3] | R. Buttaux (1951). L’analyse bactériologique des eaux de consummation. |
| [4] | M.O. Mizier (2005). La mesure de turbidité un paramètre essentiel pour l’eau potable. |
| [5] | C. Netter (1998). Détermination automatisé de la DBO5. |
| [6] | Organisation Mondiale De La Sante (OMS) «l’utilisation des usées recommandations visées sanitaires» OMS Genève, 1989. |
| [7] | J. Rodier (1956). Détermination de la dureté dans les eaux par la méthode au complexon III. |
| [8] | T.J. Cardwell et al (1990). Determination of calcium in waters milk and by discontinuous-flow analysis, Analyst, 115: 1235. |
| [9] | J.F. Van Standen et S.I. Tlowana (2001). Spectrometric determination of chloride. |
| [10] | P. Breuil et al (1998). Analyse du cuivre et de zinc dans les effluents industriels par spectrometrie UV-visible, Analysis, 26:8. |



 Among the 12 sacred lakes we call lake ANJAVIBE, this lake is located in the borough of BEMAGNONDROBE. It is located in the north of the island. The population in this borough uses this lake as drinking water. So this for cella that we draw from my research aims to analyze the physicochemical parameters and the quality control of this lake called ANJAVIBE to make known the situation of this lake is what drinkable or not.
Among the 12 sacred lakes we call lake ANJAVIBE, this lake is located in the borough of BEMAGNONDROBE. It is located in the north of the island. The population in this borough uses this lake as drinking water. So this for cella that we draw from my research aims to analyze the physicochemical parameters and the quality control of this lake called ANJAVIBE to make known the situation of this lake is what drinkable or not. The study of these parameters is different from other research,This study consists of four parts, beginning with the bibliographic synthesis and then the results of measurement for the physico-chemical and microbiological analyzes, followed by the interpretation and the discussion of this result, and ends with the conclusion.
The study of these parameters is different from other research,This study consists of four parts, beginning with the bibliographic synthesis and then the results of measurement for the physico-chemical and microbiological analyzes, followed by the interpretation and the discussion of this result, and ends with the conclusion. Recommendation of the EU
Recommendation of the EU Recommendation of the EM
Recommendation of the EM Metals: may exist in water form trace.Pollutions: these very dangerous in water it presents in microbe.Quality standardThe standard of qualities is referred to by the European Union (U.E) recommendation and the World Health Organization (WHO).
Metals: may exist in water form trace.Pollutions: these very dangerous in water it presents in microbe.Quality standardThe standard of qualities is referred to by the European Union (U.E) recommendation and the World Health Organization (WHO).

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

















