-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2017; 7(4): 110-114
doi:10.5923/j.re.20170704.04

Comparative Analysis of Three Borehole Water Sources in Nsukka Urban Area, Enugu State, Nigeria
Okoro N., Omeje E. O., Osadebe P. O.
Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Nigeria
Correspondence to: Omeje E. O., Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

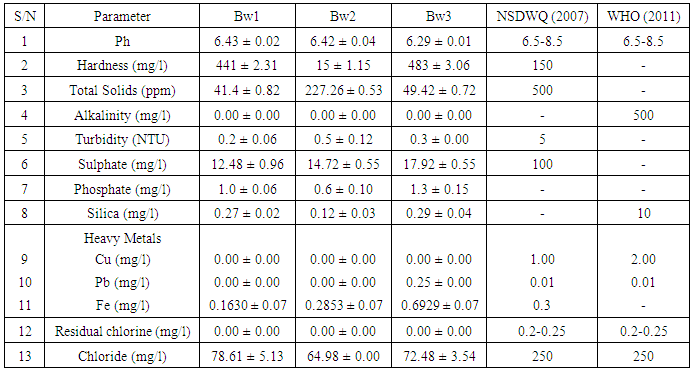
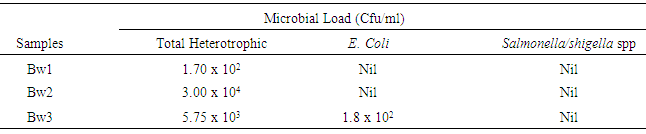
In an attempt to probe the quality of borehole water samples in Nsukka urban area of Enugu State, Nigeria, samples were collected from three locations within the area and analysed for some physico-chemical and microbial parameters, which were compared with the Nigerian Standard for Drinking Water Quality (NSDWQ) and the World Health Organization (WHO) standard for compliance evaluation. The physicochemical parameters include; pH, Hardness, Total Solids, Alkalinlity, Turbidity, Sulphate, Phosphate, Silica, Cu, Pb, Fe, Residual Chlorine and Chloride with results ranging from 6.29-6.43, 15-483mg/l, 41.4-227.2mg/l, 0.00-0.00mg/l, 0.2-0.5NTU, 12.48-17.92mg/l, 0.6-1.3mg/l, 0.12-0.29mg/l, 0.00-0.00mg/l, 0.00-0.25ppm, 0.1630-0.2853ppm, 0.00-0.00mg/l and 64.98-78.61mg/l respectively. All the physicochemical parameters were within the standard limits recommended by WHO and NSDWQ, except for the following; pH which was slightly below the standard limit (6.5-8.5), Hardness for Bw1 and Bw3 which were above the NSDWQ standard limit of 150mg/l and Pb which was present in Bw3 above the limit of 0.01ppm. High values were obtained for Total Heterotrophic count of coliform bacteria with values ranging from 1.70 x 102 - 3.00 x 104Cfu/ml, which are well beyond the NSDWQ stipulation of 10Cfu/ml. Escherichia coli was detected in Bw3 having counts of 1.8x 102Cfu/ml, which is grossly above the NSDWQ stipulated limit of 0Cfu/ml. Salmonella spp. and Shigella spp. were not detected in any of the borehole water samples.
Keywords: Borehole Water, Physico-chemical parameters, Microbial Analysis
Cite this paper: Okoro N., Omeje E. O., Osadebe P. O., Comparative Analysis of Three Borehole Water Sources in Nsukka Urban Area, Enugu State, Nigeria, Resources and Environment, Vol. 7 No. 4, 2017, pp. 110-114. doi: 10.5923/j.re.20170704.04.
1. Introduction
- Water is a natural chemical substance which consists of the elements; hydrogen and oxygen in the ratio of two is to one (2:1). It is indispensable for man’s existence on earth as about two-thirds of the human body consists of water and requires between one to seven (1-7) liters of water per day for its appropriate functioning to avoid dehydration [1, 2]. Broadly, the demand for water by man can be divided into three (3) major categories; domestic, industrial and agriculture [3]. A guaranteed supply of water for these purposes greatly improves the health, social and economic facets of human life [4]. The quality of water depends on its physical, chemical, and biological characteristics, which determines its utility for different purposes [5]. Water fit for human consumption is referred to as potable or drinking water and should be of safe quality, which entails that it does not present any significant health risk over life time consumption [6]. Drinking water schemes conventionally employed include surface water (rivers, streams, lakes, etc.) and groundwater (boreholes and wells). However, there is a rising dependence on groundwater due to increasing contamination of surface water and as it is believed to be purified as water moves down the bedrock [7].Although water is essential for life, it also remains an important source of disease transmission and a major cause of mortality in developing countries because of limitations in access and quality [8]. Drinking water can become contaminated with foreign matter such as pathogenic bacteria (Salmonella typhi, Shigella dysentariao, Escherichia coli, Klebsiella pneumonia etc.), chemical substances (fertilizer, pesticides, metals etc.) and industrial effluents or other wastes, which deteriorates its quality; rendering it unfit for its intended use [8, 9]. According to the WHO drinking water fact sheets, contaminated water can transmit diseases such diarrhea, cholera, dysentery, typhoid, and polio. Also, contaminated drinking-water is estimated to cause 502, 000 diarrheal deaths each year [10]. This makes it expedient to ascertain the safety of drinking water by determining the extent to which it conforms to the standards set by regulatory bodies.In Nigeria, access to safe drinking water and hygienic sanitation facilities still pose serious challenge to its rapidly growing population of more than 174 million people [11]. In Nsukka Urban area, a popular town in Enugu State, Nigeria, there is an absence of surface water, causing a heavy reliance on underground water as the main source of potable water supply, with boreholes serving as the conventional scheme employed. Dwellers either go to purchase water in plastic gallons or tanker delivery to homes is employed.This study is aimed at evaluating the physico-chemical and microbial quality of borehole water sources in Nsukka Urban area, comparing them with set regulatory standards.
2. Materials and Methods
- Three borehole water samples, labeled Bw1 (Borehole Water 1), Bw2 (Borehole water 2) and Bw3 (Borehole water 3) were randomly collected from Nru, Nkpunano, and Ihe/Owerre Areas that make up Nsukka Urban Area. The samples were collected using sterile 75cl plastic containers and taken to the laboratory for physicochemical and microbial analysis. The physicochemical parameters were determined using known standard methods and analysis were carried out in triplicates [12, 13]. The pH was determined using the Jenwey pH meter (model 3510), Turbidity with a turbidity meter, and Total Solids by gravimetry. Chloride was determined by Mohr’s Argentometric method, Residual Chlorine by Iodometric method and Total Hardness by Complexometric (EDTA) titration. Total Alkalinity and Sulphate were determined by titrimetry, while Phosphate and Silica were determined by Colorimetry. The metals were determined by AAS (model AA-7000). The microorganisms were isolated using the following media: Nutrient agar, Salmonella/shigella agar and Eosine methylene blue agar which were all prepared according to the manufacturer’s specification and sterilized by autoclaving at 121oC for 15 minutes. The total coliform counts were carried by the standard plate count technique, while Salmonella/Shigella and E.Coli were determined using Salmonella/Shigella and Eosin Methylene Blue agar respectively by pour plate technique. The isolated microorganisms were subjected to characterization tests as described by Chessbrough [14]. Gram staining and biochemical tests (Coagulase and Catalase tests) were used to characterize the isolates.
3. Results and Discussion
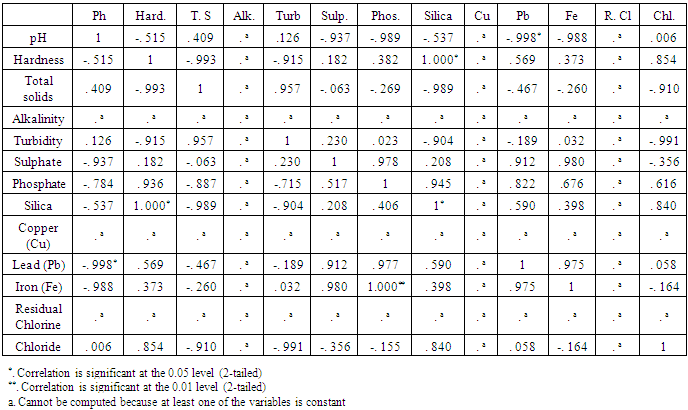
- The results for the physicochemical parameters and microbial analysis of the borehole water samples collected from the selected locations in Nsukka Urban Area are presented in Table 1 and 2 respectively. Table 3 presents the correlation matrix for the physicochemical parameters determined using the Karl Pearson’s correlation Analysis. The Correlation matrix was used to establish the relationship between the various physicochemical parameters of the borehole water samples.Generally the pH of the samples tended to be slightly acidic, with values varying from 6.29-6.43 which fell slightly below the WHO and NSDWQ permissible limit of 6.5-8.5. Consequently, Alkalinity values for the borehole water samples were 0.00mg/l, which is within the WHO permissible limit. This trend was also reported in previous studies carried out at Nsukka and Aba, Abia State [15, 7]. Through previous studies, acidic water has been shown to have no negative implication on human health, but pH values below 4 can lead to irritation due to corrosive effect. However, alkaline water has been proven to have some health benefits as it aids calcium retention thereby improving bone health [16]. Deviations in the pH value of water solutions from 7 are principally due to hydrolysis of salts of strong bases and weak acids or vice versa, also dissolved gases such as carbon dioxide, hydrogen sulphide, and ammonia affect the pH of water [2]. In this study, pH showed a strong negative correlation with Sulphate (-0. 937), Phosphate (-0. 989), Lead (-0. 998) and Iron (-0. 988) which implies that as the values of Sulphate, Phosphate, Lead, Iron, either increases or decreases the pH values tend towards the opposite direction almost correspondingly. This is so because the presence of sulphate and phosphate ions in water tends to increase its acidity thereby causing a decrease in pH value.The Hardness, Total Solids, and Turbidity values ranged from 15-483mg/l, 41.4-227.26mg/l, and 0.2-0.5mg/l respectively. The Total Solids and Turbidity values were within the standard limits of 500mg/l and 5mg/l respectively, while the hardness values were above the NSDWQ limit of 150mg/l except for Bw2 with a value of 15mg/l. According to WHO (2011) no health-based guideline value is proposed for hardness, as it is not considered to be of major health concern at levels found in drinking water, though it may affect its aesthetic acceptability [17]. Hardness is as a result of the presence of calcium or magnesium salts in water. The occurrence of calcium can be beneficial for the growth of children whereas high intake of magnesium causes a change in bowel habits (diarrhea) [18, 19]. Also, turbidity per se is not necessarily a threat to health, but can have a negative impact on consumer acceptability as a result of visible cloudiness, and can be an important indicator of possible presence of contaminants that would be of concern to health, when of high values [17]. The trend in turbidity is in agreement with studies conducted in Owerri, Imo State on borehole water samples [20]. Hardness showed a strong positive relationship with chloride (0.854) and silica (1.000), which is expected as hardness can be caused by the presence of calcium and magnesium chlorides and by the occurrence of other dissolved metals in water [19]. Total Solids showed a strong positive correlation with Turbidity (0.957) and a strong negative correlation with Silica (-.989) and Chloride (-0.910), while Turbidity had a strong negative correlation with Silica (-0.904) and Chloride (-0.991). Since Turbidity is caused by the presence of suspended particles or colloidal matter in water, an increase or decrease in the concentration of total solids will lead to a corresponding decrease or increase in turbidity [17]. The values for Sulphate ranged between 12.24 - 17.92mg/l which are low compared to the standard recommended limit of 100mg/l stipulated by NSDWQ. These values have also been reported in a similar studies carried out in Aba [17]. High concentrations above the recommended limit in drinking water are known to cause dehydration and gastro-intestinal irritation [3]. Sulphate showed a high positive relationship with Phosphate (0.978), Lead (0.912) and Iron (0.980). Given that Sulphate anions are usually associated with cations in water to form moderately soluble salts, a positive relationship can be anticipated with Lead and Iron [17].Low Phosphate values were obtained ranging from 0.4 - 1.3mg/l. This is also as reported in a study carried out in Benin City, Edo State (21). It showed a strong positive correlation with Lead (0.822) and Silica (0.945). The concentration of Silica fell below the permissible limit (10mg/l) as recommended by WHO, with values ranging from 0.12-0.29mg/l. Silica showed a moderately strong positive correlation with Chloride (0.840). Residual Chlorine was not detected (0.00mg/l) in the borehole water samples which are below the lower limit (0.2-0.25mg/l) stipulated by NSDWQ and WHO. This is an ideal result as the presence of excess residual chlorine in water has adverse health effects; by-products of chlorination can set off the production of free radicals, causing cell damages associated with cancer cases. The results for Chloride ranged between 64.98 - 78.61mg/l, which is within the permissible limit stipulated by NSDWQ and WHO (250mg/l). This was also observed in a similar study carried out in Aba, MOUAU, and Nnewi [17, 22, 23].Chloride has not been reported to have a significant negative health impart. Moreover, a daily dietary intake has been recommended by WHO for adults (9mg) and individuals up to 18yrs (45mg). Heavy metal analysis showed that Copper (Cu) was not detected in any of the samples, while Lead was detected in Bw3 (0.25ppm) being above the permissible limit of 0.01ppm stipulated by NSDWQ and WHO. This could be as a result of other industrial activities (e. g lead soldered cans, pipes and from petrol), carried out within the area. The concentration of Iron ranged from 0.1630 - 0.6929ppm, which is within the limit stipulated by NSDWQ (0.3mg/l) except for Bw3 with a concentration of 0. 6929mg/l. Iron (Fe) and Copper (Cu) have been categorized as being essential for human life at moderate levels. Lead (Pb) is one of the most widely recognized metal toxins which could cause teratogenic effects, dysfunctions in the kidneys, joints, and reproductive systems. It could also cause acute damage to the central nervous system (CNS) and peripheral nervous system (PNS) [24]. Pb showed a high positive correlation with Iron (0.975).
|
|
|
4. Conclusions
- The results of this study showed that all the physico-chemical parameters of the borehole water samples were within the standard limits recommended by WHO and NSDWQ, except for the following; hardness and the pH of borehole water samples, which were slightly below the standard, and Lead which was observed in Bw3. The differences observed in the concentration of these water quality parameters from one borehole to another can be attributed to climatic, geographic, and geologic variations between the three (3) areas of sample source [7]. Also, disparity in the hygienic condition of the boreholes plays a major role, as well as human and industrial activities. Based on the results for the microbial analysis borehole water samples could also be considered satisfactorily safe for consumption, even though they showed high total coliform counts, because the parameter in itself does not present a risk to human health. An exception however, is observed in Bw in which E. coli was detected and isolated, rendering them unsafe for human consumption. In general, it can be concluded that most of the water samples under study are safe for human consumption, except for water sample from Bw3, which were shown to be contaminated with lead and the faecal indicator organism, E. coli. From the foregoing, it should not be automatically assumed that water packaged in sachets, as well as borehole water sources are safe hence, it is necessary to treat water from borehole before consumption to prevent the spread of water borne diseases to ensure that the health of the community is protected.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML