Tambeke N. Gbarakoro, Gloria O. Anianu
Department of Animal and Environmental Biology, University of Port Harcourt, Choba, Nigeria
Correspondence to: Tambeke N. Gbarakoro, Department of Animal and Environmental Biology, University of Port Harcourt, Choba, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The Contributions of Soil Microarthropods to Ecosystem recovery in Oil-Polluted Habitats was investigated for six months during the dry season, November 2012 – April 2013, in Tai Local Government Area in Rivers State, Nigeria. Three study habitats were selected based on the timing of the spill before the commencement of the study. Habitats polluted and re-polluted 1 year pre-study (Gio), 8 years pre-study (Norkpo I) and an unpolluted cassava farm (Norkpo II)referred to as frequently polluted, non-frequently polluted and unpolluted, respectively were selected. Soil samples were collected monthly, from depth ranges: 0-5cm, 5.1-7.5cm, 7.6-10.0cm, 10.1-12.5cm, 12.6-15.0cm, 15.1-20.0cm, with an 8.5cm bucket soil auger. Soil microarthropods were extracted using the Modified Bukard Model of the Berlese-Tullgren Funnel. Recovery Status was determined by arthropod species richness and abundance, indicator species and soil profile population dynamics. Total Hydrocarbon Content (THC) and soil edaphic factors were measured. The mean Total Hydrocarbon Content (THC) was higher at Gio (480mg/kg). The average pH, temperature and moisture content were highest at Norkpo II, Norkpo I and Gio respectively. The highest moisture content was recorded in the month of December at unpolluted followed by frequently polluted habitats. Recovery status indicated a total of 980 soil microarthropods collected with the lowest at Gio (87) and the highest at Norkpo II (660). The 15 species of soil microarthropods collected belonged to Collembola (1) and two orders of mites: Mesostigmata (3) and Cryptostigmata (11). Norkpo II had the highest number of species (15) while Gio had the lowest number of species (6). There were two species of microarthropods (Mixacarus sp. and Uropodide sp.) that were absent in Gio but recovered at Norkpo I. The density of recovered soil microarthropods was more at 0-10.0cm depth than 10.1-20.0cm depth indicating that recovery with depth was attaining normalcy as observed in the control habitat. Statistical analysis between the abundance of recovered species and yet to be recovered species was (F= 5.11; df= 5; p> 0.05) and is significant. The high THC values recorded contributed drastically to the low number of species richness and abundance in the polluted habitats. Frequent hydrocarbon contamination increases moisture content of impacted soil habitat and reduces abundance of soil microarthropods. Organisms that were absent as a result of the pollutant are referred to as indicator species while those that persisted and tolerated the pollutant are monitor species. About 9 years after the spill (Norkpo I) species richness and abundance of soil microarthropods have not been fully recovered. Apparently it will take longer years of microbial activities for the ecosystem to return to predisturbance levels therefore other methods of bioremediation should be employed.
Keywords:
Soil microarthropods, Recovery status, Species richness, Abundance, Oil pollution, Edaphic factors, Soil profile
Cite this paper: Tambeke N. Gbarakoro, Gloria O. Anianu, Contributions of Soil Microarthropods to Ecosystem Recovery at Tai Communities, Rivers State, Nigeria, Resources and Environment, Vol. 6 No. 6, 2016, pp. 136-142. doi: 10.5923/j.re.20160606.06.
1. Introduction
Soil microarthropods are arthropods that creep on and inside the soil [1] whose sizes are less than 2mm and do not permit the detailed study of their morphological features with the naked eye [2]. These microarthropods are small invertebrates which mainly inhabit soil surfaces, interphases, and top soil, feeding on decaying vegetation, bacteria, fungi, protists and nematodes [3]. Soil microarthropods play key roles in the decomposition and mineralization of dead organic matter, enhancement of soil fertility, regulation of microbial populations, soil formation and are indicators of soil health and ecosystem recovery. Ecosystem recovery occurs when a contaminated, degraded, damaged or destroyed ecosystem is made artificially or naturally to return to those features it possessed before it was contaminated. Recovered soil microarthropods are soil microarthropods that left a particular habitat as a result of the pollutant but returned when the habitat had recovered from the effects of the pollutant. The reverse of this is the unrecovered soil microarthropods. Ecosystem recovery by natural or artificial processes is a biological process that degrade, breakdown, transform and/or essentially remove contaminants from soil or water. This is referred to as Bioremediation, and when it occurs without human intervention other than monitoring is often called natural attenuation, which relies on natural conditions and behavior of soil microorganisms that are indigenous to the soil [4]. The process of bioremediation can be enhanced through the activities of monitor species which are generally insensitive and tolerant to the stress. In an earlier study on ecosystem recovery determined by recovered species and recovery trend, three species of the eleven Cryptostigmatids found in the unpolluted habitat and four Mesostigmatid were absent from the habitat polluted 1year but recolonized habitat polluted 3years pre-study. In the habitat polluted 6years pre-study, an additional two and one species that belong to Cryptostigmata and Mesostigmata recolonized [5]. On recovery trend, 8years after the oil spill, species richness in Cryptostigmatid and Mesostigmatid mites were not fully restored indicating that the recovery status of the impact ecosystem was partial, in spite of the fact that a recovery trend was enhanced by the reduction in the Total Hydrocarbon level under natural conditions due probably to the contributions of monitor soil microarthropods [5].This present study is conducted to ascertain the contributions of soil microarthropods to the recovery status of oil impacted ecosystem through the following indices:Ÿ Soil profile population dynamics.Ÿ Species richness and abundance.Ÿ Bio indicator species and recovery trend.
2. Materials and Methods
2.1. Study Area
The study was conducted at Gio and Norkpo, Tai Local Government Area (L.G.A), Rivers State, Nigeria. The area is bounded by Oyibo Local Government Area (L.G.A) to the North East, Eleme Local Government Area (L.G.A) to the North West, Khana Local Government Area (L.G.A) to the East and Gokana Local Government Area (L.G.A) to the South [5]. The study sites in each community were approximately 3500m2 of secondary forest in the low-land rainforest belt. Tai L.G.A is located in an area that has rainy and dry seasons; the rainy season lasts from March to October and the dry season lasts from November to February. Tai L.G.A covers an area of 159km2 and at 2006 census it had a population of 117,797 [6].
2.2. Study Site
There were three sites which were replicated four times in a randomized complete Design, engaged in this study. The frequently polluted site is located at Gio, non-frequently polluted site located at Norkpo I and unpolluted site located at Norkpo II (Fig 1).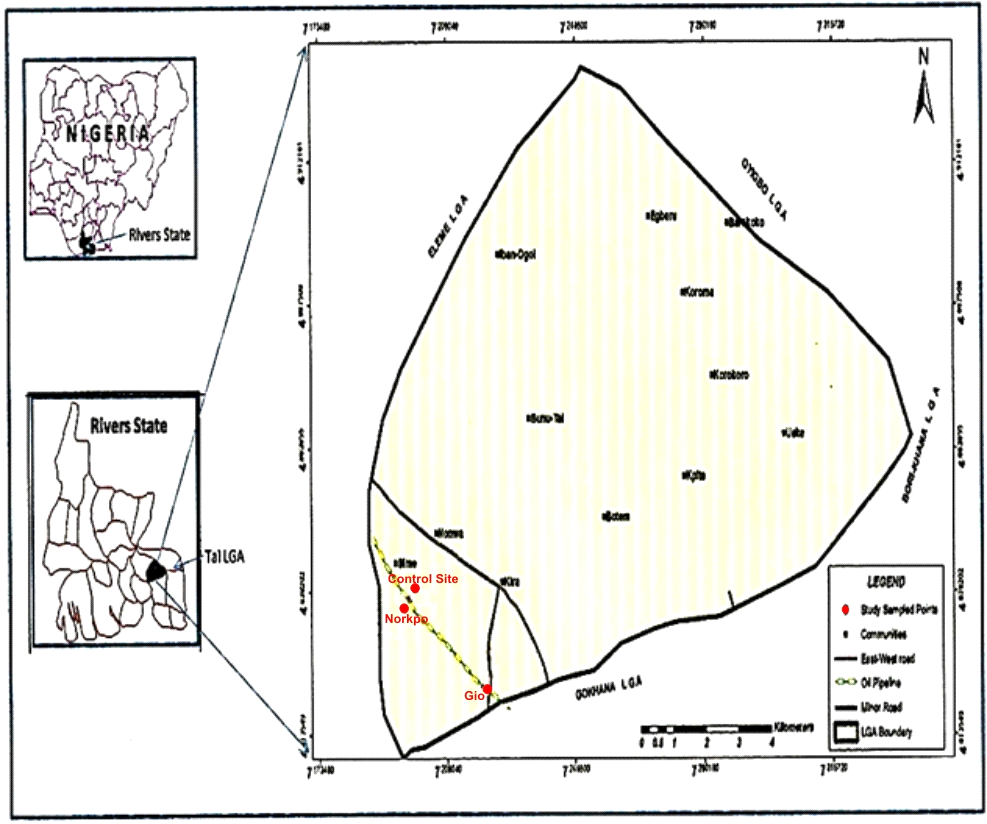 | Figure 1. Study Villages in Tai LGA, Rivers State (Gbarakoro et al., 2010) |
The frequently polluted habitat is an abandoned farmland located on (04° 41.91′N, 7° 14.86′E) on the South- East of the map (Fig 1) and polluted with petroleum oil spill on several occasions [7]. It was polluted in 2004, remediated in 2010 and polluted 2011, a year before the commencement of the study. The Total Hydrocarbon level was 480mg/kg (plate 1). | Plate 1. Gio Habitat (Polluted 1 year pre-study) |
The Non-frequently polluted habitat is an abandoned farmland polluted about 8years (2004) prior to the commencement of the study. It is located on (04° 44.13′N, 7° 12.30′E) on the South West of the map (Fig 1). The Total Hydrocarbon level was 150mg/kg and no remediation has taken place (plate 2). | Plate 2. Norkpo I (Polluted 8 years pre-study) |
The unpolluted habitat which served as the control is located on the South-West (04° 44.17′N, 7° 12.30′E) on the South-West of the study map (Fig 1). The Total Hydrocarbon level is 15mg/kg (Plate 3). | Plate 3. Norkpo II (Control Habitat: An unpolluted cassava farmland) |
2.3. Extraction
The modified Bukard model of the Berlese-Tullgren funnel was used for extraction [8]. Description of the extractor complex and extraction procedure has been documented [9]. Extraction processes lasted for 7days. The extracts containing soil microarthropods were placed in Petri-dishes, under a dissecting microscope at the Entomology Research Laboratory, Department of Animal and Environmental Biololgy, University PortHarcourt, where they were sorted, and identified using identification keys (10; 11; 12) and type-specimens.
2.4. Determination of Edaphic Factors (Moisture, Temperature, pH)
2.4.1. Soil Moisture Content
50g of soil sample was collected from 0-10cm depth of each site and weighed with OHAUS portable scale scout II electrical weighing balance manufactured in China to determine soil moisture content. Samples were wrapped in Tower foil paper, labeled and placed in B and T laboratory Thermal Equipment (oven) manufactured in England for 24hours. 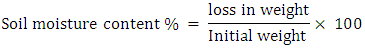
2.4.2. Soil Temperature
Soil temperature readings were taken by inserting the thermometer (Quick fit mercury-in-glass) into the soil to a depth of 10cm for 5minutes before taking the reading in degree Celsius (°C).
2.4.3. SOIL pH
20g of soil was sun dried and placed in a 50ml beaker. 20ml of distilled water was added, stirred occasionally with a glass rod and allowed to stand for 30minutes to determine the soil pH. The electrode of each Equip-Tronics digital pH meter (model EQ-610) was then inserted into the solution and pH readings recorded. Edaphic factor readings were recorded monthly.
2.5. Determination of Total Hydrocarbon Level
The 1985 ASTMD 3921 (modified) method was used to determine the THC. 1g of sieved soil sample was extracted in 10ml chloroform (CC13H), in a glass test tube. The CC13H layer was taken with a clear test tube and dehydrated by adding a spoonful of anhydrous sodium sulphate. The clear extracted solution was absorbed at 420mm wavelength with thermospectromic spectrophotometer.
2.6. Sampling
Soil samples were collected with an 8.5cm bucket-type soil auger from six depth ranges; 0-5.0cm, 5.1-7.5cm, 7.6-10.0cm, 10.1-12.5cm, 12.6-15.0cm and 15.1-20.0cm from all the sites. During each collection, the auger was pushed into the soil by applying pressure on the handle with both hands, and then rotated clockwise and anticlockwise direction to obtain the soil from each depth range. Each sample was placed in polythene bag, labeled and taken to the laboratory for analyses.
3. Results
3.1. Ecosystem Recovery Determined by Species Richness
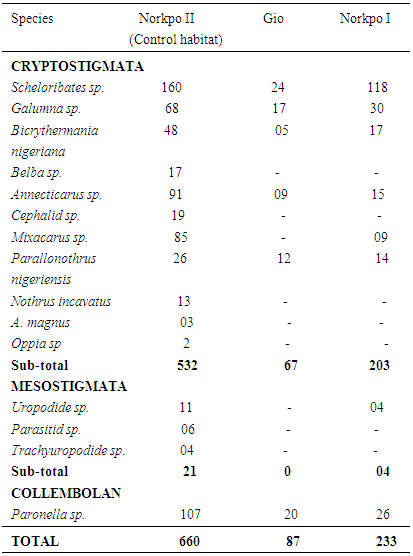
A total of 15species of soil microarthropods belonging to three orders; Cryptostigmata, Mesostigmata and Collembola were recorded with 11, 3, and 1 respectively (Table 1). The frequently polluted habitat recorded a total of 6 species; Cryptostigmata (5), Scheloribates sp; Galumna sp; Bicrythermania nigeriana, Annecticarus sp; Parallonothrus nigeriensis and Collembola (1) Paronella sp. In the non-frequently polluted habitat, a total of 8 species comprising all the species that occurred at the frequently polluted habitat and two other species – Mixacarus sp; and Uropodide sp; (Table 1). In the non-polluted habitat, a total of 15species were recorded, and they include all that occurred in the polluted habitats and 7 others-Belba sp; Oppia sp; Nothrus incavatus, Cephalid sp; Parasitid sp; and Trachyuropodide sp.
3.2. Ecosystem Recovery Determined by Bioindicators
At the end of the study, 7 species that were absent in the frequently polluted habitat were present in the unpolluted habitat (Table 1). The 7 species are indicators of the severity of the spill over the long period.Table 1. Species Richness and Abundance of Mites and Collembola at Gio, Norkpo I and Norkpo II (Control Habitat)
 |
| |
|
3.3. Ecosystem Recovery Determined by Species Abundance
A total abundance of 87 and 220 individual monitor microarthropods were collected from frequently polluted and non-frequently polluted habitats respectively. Among the monitor species, the most abundant were Scheloribates and Galumna species while Bicrythermania nigeriana and Annecticarus species recorded the least. A total of 980 individuals of soil microarthropods were collected from both the polluted and unpolluted habitats with the latter recording the highest and frequently polluted the least abundance. A total of 660 individuals of soil microarthropods representing 67% were collected from the unpolluted habitat, and 87 individuals representing 9% were collected from the frequently polluted habitat while 233 individuals representing 24% was collected from the non-frequently polluted habitat.
3.4. Total Hydrocarbon Content in the Three Habitats
The Total Hydrocarbon Content from the three habitats varied greatly. The frequently polluted habitat had 480mg/kg, non-frequently polluted habitat had 150mg/kg while the unpolluted habitat had 15mg/kg. Table 2. Relative Abundance of Indicator Species in the Polluted Habitats (Gio and Norkpo I)
 |
| |
|
3.5. Ecosystem Recovery Determined by Soil Profile Population Dynamics
A total of 980 soil microarthropods were collected from depth ranges of 5-20cm in all the habitats. The least abundance was recorded at 15.1-20cm while the highest abundance was recorded at 5.1-10cm depth ranges in all the habitats (Table 3). At 0-10cm depth range, a total of 704 soil microarthropods were recorded and a total of 276 recorded from depths of 10cm and above.Table 3. Soil Microarthropods at the Three Depth Ranges in the Three Habitats
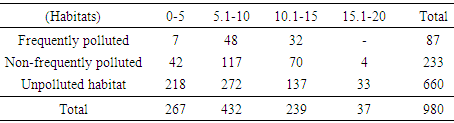 |
| |
|
Among the three orders of soil microarthropods; Cryptostigmata (Oribatida), Mesostigmata (Gamisida) and Collembola (Springtails), a total abundance of 802, 25 and 153 were collected in the respective groups. Frequently polluted habitat recorded a total abundance of 67 Cryptostigmatid mites, zero Mesostigmatid mites and 20 Collembolans. Non-frequently polluted habitat recorded 203 Cryptostigmatid mites, 4 Mesostigmatid mites and 26 Collembolans (Tables 4-6). The unpolluted (control) habitat recorded a total abundance of 532 Cryptostigmatid mites, 21 Mesostigmatid mites and 107 Collembolans.Table 4. Abundance of Cryptostigmata at Various Depths during The Study
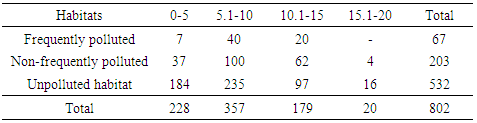 |
| |
|
Table 5. Abundance of Mesostigmata at Various Depths during the Study
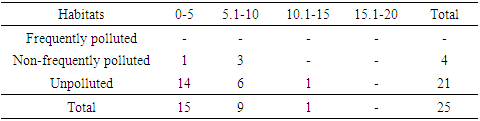 |
| |
|
Table 6. Abundance of Collembola at Various Depths
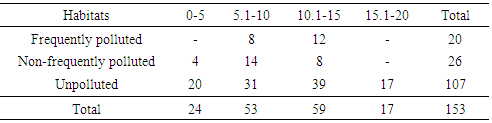 |
| |
|
At 15.1-20cm depth range, all the three orders of soil microarthropods recorded the least abundance in all the habitats with Cryptostigmata (20), Mesostigmata (zero) and Collembola (17). At 5.1-10cm depth, highest abundance was recovered in all the habitats with Cryptostigmata (375), Mesostigmata (15) and Collembola (53) (Table 4-6).
4. Discussion
The contributions of soil microarthropods to ecosystem recovery measured by species richness indicated that two species; Mixacarus sp and Uropodide sp that were absent in the frequently polluted habitat recolonized the non-frequently polluted habitat. The absence of seven species in the frequently polluted habitat has caused delayance in the recovery of the ecosystem. The classification of these species as indicator agrees with the description that they are organisms that are susceptible to the pollutants and indicate a significant level of contamination [13]. The indicator species recorded in this study agrees with those recorded by [5] except Nothrus incavatus and Parasitid species which were collected in the rainy season and not in this study. There were more indicator species in the unpolluted habitat than the non-frequently-polluted habitats as 15 and 2 species respectively were present. These indicate that non-frequently polluted habitat is gradually recovering from the effects of the spill.Six species of soil microarthropods were recorded in both polluted habitats. The species tolerated the oil pollutant and persisted in the ecosystem as they were able to survive the harsh conditions. The species included; Scheloribates, Galumna sp; Bicrythermania nigeriana, Annecticarus sp; Parallonothrus nigeriensis and Paronella sp. The presence and activities of these monitor species naturally contribute to the process of bioremediation and consequently enhance a faster recovery of the ecosystem. These monitor species agrees with the report that they are organisms that have the ability to withstand the stress caused by oil pollution either by tolerating high levels, accumulating or excreting the pollutant ([13]. These monitor species agrees with those recorded by [14].Abundance of monitor species is a major contribution to ecosystem recovery because the ecosystem that recorded high population is an indication of improvement of the health and recovery status of the ecosystem. This is observed in non-frequently polluted habitat, especially when compared with unpolluted habitat whose abundance of monitor species was 500.The abundance of recovered species (Mixacarus and Uropodide) at non-frequently polluted habitat was 13 against zero in the frequently polluted habitat. This implies that the non-frequently polluted habitat is gradually recovering (Table 2).Recovery by abundance indicates that no appreciable recovery is taking place in frequently polluted habitat. Inspite of high moisture content recorded in the month of December in this same habitat recovery by abundance was hampered by high concentration of total hydrocarbon content. Soil pH and temperature varied slightly across the habitats, but there was no appreciable influence on the abundance of soil microarthropods. The highest moisture values in this study was recorded in the month of December; 11.6% (frequently polluted), 4% (non-frequently) and 14% (unpolluted or control) habitats (Fig 2). Abundance of soil microarthropods in the month of December was 14, 55 and186 in frequently, non- frequently and unpolluted habitats respectively.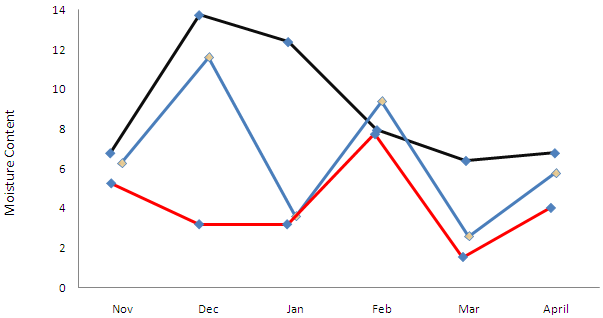 | Figure 2. Monthly Soil Moisture Content in Frequently Polluted (Blue), Non-Frequently Polluted (Red) and Unpolluted (Black) Habitats |
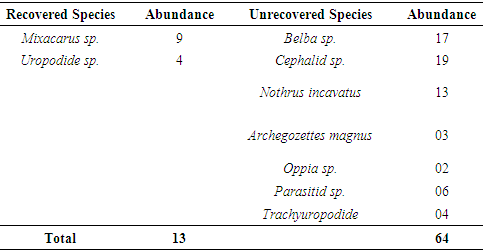
The reduction in concentration of pollutant was probably caused by the activities of the soil microarthropods especially monitor species and the length of time between the spill and the commencement of study. The frequency of occurrence affected the trend of recovery of the ecosystem in the frequently polluted habitat. This agrees with the report that the frequency of disturbance prolong the trend of ecosystem recovery [15] as indicated by frequently polluted habitat, but in the absence of further disturbance, many ecosystems tread back towards pre-disturbance conditions [16] as indicated by non-frequently polluted habitat. There is a significant difference between the abundance of recovered species and yet to be recovered species (F=5.11; df=5; P>0.05). This indicates that the ecosystem is yet to attain full recovery. Hydrocarbon increases the moisture of the soil, as the higher the THC, the higher the moisture and the lower the abundance. This is the situation in the frequently polluted habitat where high concentration and severity of THC delay the recovery of the habitat, unlike in the non-frequently polluted habitat where the THC values was reduced, the moisture content low and abundance higher. Frequent hydrocarbon contamination increases moisture content of impacted soil habitat and reduces abundance of soil microarthropods where its concentration is high, thereby reducing the rate of recovery of the habitat. This agrees with the report that high THC may cause oxygen deprivation and subsequent death of soil fauna due to asphyxiation [17]. This is contrary to the situation in the unpolluted habitat where the lower the THC, the higher the moisture content, and the higher the abundance of soil microarthropods.Two species: Mixacarus sp. and Uropodide sp; that were absent in frequently polluted habitat but recovered at non-frequently polluted habitat recorded an abundance of 13 individuals below 10.0cm depth. The unrecovered abundance of these species were Mixacarus sp (76) and Uropodide sp (7). The number of species collected from this depth at non-frequently polluted habitat shows that 7 species: Belba sp., Cephalid sp., Nothrus incavatus, Archegozettes magnus, Oppia sp; Parasitid sp; and Trachyuropodide sp are yet to be recovered. These species recorded a total abundance of 64 at the unpolluted habitat. This indicates that generally, only 22.2% of the species have been recorded. The total abundance of these two species was 96 (control) and 13 (non-frequently polluted) habitats. The percentage abundance of recovered species was 13.5% indicating that 13.5% of the total abundance of the two species in the impacted habitat has been recovered naturally over 8years of post pollution.
5. Conclusions
In conclusion, the present study has shown that; Soil microarthropods facilitate the process of recovery of the petroleum oil impacted ecosystems. This is because certain species in this study are insensitive and tolerant of the contaminant, feeds on it and degrade or reduce the concentration of the contaminant. The species are monitor species whose presence, abundance and activities can be used to assess the level of ecosystem recovery.There is a provision of information on the “health status” of the contaminated ecosystem indicating the “health recovery status” of the ecosystem. This is evident by the disappearance of some species from the ecosystem due to the severity of the contaminant. Those species are bio indicator species and were more indicated in the frequently polluted habitat. However, these species were more abundant in the non-frequently polluted habitat indicating that this habitat is “health recovery status” higher than the frequently polluted habitat.The disturbed mites though not fully with regard to abundance re-occupied their normal depths in the soil profile, but their population changes in the profile could serve as bio indicator of ecosystem recovery from natural and anthropogenic environmental stressors in the impacted ecosystems.There is a relationship between the biological and chemical analyses as the Total Hydrocarbon levels of the habitats support the findings of monitor and indicator species which indicate that recovering of the ecosystem is gradually taking place. The frequency of occurrence affected the trend of recovery as frequently polluted habitat is yet to attain the species richness possessed by non-frequently polluted habitat.
References
| [1] | Iloba, B. N. and Ekrakene T. (2008). Soil microarthropods associated with mechanic workshop soil in Benin City, Edo state Nigeria. Research Journal of Agriculture and Biological Sciences. 4(1): 40-45. |
| [2] | Badejo, M.A. (1998). Quick Notes on soil Microarthropod studies. Department of Zoology, Obafemi Awolowo University, lle-Ife, Nigeria, 35pp. |
| [3] | Holowko, P. (2010). Berlese funnel. Invertebrate biodiversity in the soil. Juniata College. www.juniata.edu/services/science. |
| [4] | Dana, L. D. and Bauder, J. W. (2006). Biobasics: the science and the issues; a general essay on bioremediation of contaminated soil. http://www.biobasics.gc.ca/english/view.asp?x=741. |
| [5] | Gbarakoro, T. N., Okiwelu, S. N., Badejo, M. A., Umeozor, O. C. (2010). Soil Microarthropods in a Secondary Rainforest in Rivers State, NigeriaSeasonal variations in Species Richness, vertical distribution and Density in an undisturbed Habitat. Scientia Africana 9(1)46-54. |
| [6] | National Population Commission. (2006) National Population and Housing Census. Federal Republic of Nigeria, Official Gazette. 94:2A. |
| [7] | The Ecumerical Council for Cooperate Responsibility (ECCR). (2010). Shell in the Niger Delta: a Framework for change-five case studies from civil society. www.eccr.org.uk |
| [8] | Lasebikan, B. A. (1974). Preliminary Communication on Microarthropods from a tropical rainforest in Nigeria. Pedobiologia 14: 402-411. |
| [9] | Badejo M.A. (1996). Measuring the diversity of soil micro-flora and microfauna in an area of conservation of biodiversity. In Biosphere resources or diversity conservation and sustainable development in Anglophone Africa (BRAA), assessment and monitoring techniques in Nigeria, Abeokuta, Nigeria. |
| [10] | Krantz, G. W. (1978). A manual of Acarology. Oregon State University Book Stores Incorporate. Corvallis, 509pp. |
| [11] | Norton, R. A. (1990). Acarina: Oribatida. In: Dindal, D. L. (Ed), Soil Biology Guide. John Wiley, New York. 779-803pp Olukoya, S. (2002). Sound of Shell’s fire in Ogoni. Urhobo historical Society. Retrieved 17/10/2009. |
| [12] | Woolley, T. A. (1990). Acarology: mites and human welfare. John Wiley, New York, 463pp. |
| [13] | Beeby, A. (1993). Applying Ecology. Chapman & Hall, London. 441pp. |
| [14] | Gbarakoro, T.N., Okiwelu, S.N., Umeozor, O.C, Badejo, M.A. (2011). Soil Microarthropods in a Secondary Rainforest, Rivers State, Nigeria-III-Partial Recvery after an oil spill. International Journal of Ecosystem 2(2):1-4. |
| [15] | Olukoya, S. (2002). Sound of Shell’s fire in Ogoni. Urhobo historical Society. Retrieved 17/10/2009. |
| [16] | Dale, V. H., Joyce, L. A., McNulty, S., Neilson, R. P., Ayres, M. P., Flannigan, M. D., Hanson, P. J., Ireland, L. C., Ariel, E., Peterson, C. J., Simberloff, D., Swanson, F. J., Stocks, B. J., Wotton, M. “Bioscience” (2001). Climatic Change and Forest Disturbance 51 (9): 723-734. |
| [17] | Osuji, L.C. and Onojake, C.M. (2006). Field reconnnaissannce and estimation of petroleum hydrocarbon and heavy metal contents soil affected by the Ebocha-8 oil spillage in Niger Delta, Nigeria. Journal of Environmental Management 79(2): 133-139. |







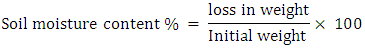

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




