-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2016; 6(3): 53-62
doi:10.5923/j.re.20160603.02

Impact of Brewery Factory Effluent on the Physicochemical Characteristics of Ikpoba River in Edo State, Nigeria
Oghenekohwiroro Edjere1, Albert C. Ibezute1, Sandra U. Ibezute2
1Department of Environmental Management and Toxicology, Federal University of Petroleum Resources, Effurun, Nigeria
2Department of Animal and Environmental and Biology, University of Benin, Benin City, Nigeria
Correspondence to: Albert C. Ibezute, Department of Environmental Management and Toxicology, Federal University of Petroleum Resources, Effurun, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

An investigation of the impact of brewery industrial effluent discharged into Ikpoba River was carried out in the months of April to September, 2012. Water samples were collected at three sites, designated as station 1 (upstream of effluent discharge point), station 2 (effluent discharge point) and station 3 (downstream of effluent discharge point) following acceptable standard and instrumentation; while physicochemical characteristics of the water sample was determined as described by APHA. The results of the water quality measurement indicated that some parameters exhibited significant spatial variation. Water temperature, total dissolved solids, electric conductivity, turbidity, chloride, sodium, potassium, sulphate, nitrate, phosphate, lead, iron, zinc and cadmium were found to be significantly higher at the discharge point (Station 2) than the upstream and downstream stations 1 and 3; while DO and pH was lower in station 2 than other stations. The parameters when compared with WHO guidelines indicated that temperature in all stations; turbidity in stations 2 and 3; nitrate, lead, iron, zinc and cadmium at station 2 exceeded the maximum allowable limit; while dissolved oxygen level at all stations were below minimum allowable limit (5mg/L) for aquatic life. All other physico-chemical parameters including biological oxygen demand were below the recommended limits in all stations. This physico-chemical regime is an indication of the deteriorating water quality of the river at the discharge point due to the effluent inflow.
Keywords: Brewery factory effluent, Ikpoba River, Water quality, Aquatic pollution
Cite this paper: Oghenekohwiroro Edjere, Albert C. Ibezute, Sandra U. Ibezute, Impact of Brewery Factory Effluent on the Physicochemical Characteristics of Ikpoba River in Edo State, Nigeria, Resources and Environment, Vol. 6 No. 3, 2016, pp. 53-62. doi: 10.5923/j.re.20160603.02.
Article Outline
1. Introduction
- Industrial effluent contamination of natural water bodies has emerged as a major challenge in developing and highly populated countries like Nigeria. Estuaries and inland water bodies, which are the major sources of drinking water in Nigeria, are often contaminated by the activities of the adjoining populations and industrial establishments [24]. River systems are the primary means for disposal of waste, especially the effluents from industries. These effluents from industries influence the pollution of the water body altering the physical, chemical and biological nature of the receiving water body [24]. Increased activities from industrial, agricultural and domestic sources have led to pollution stress on surface waters [2].Benin City is becoming fairly industrialized [14]. Although some of these industries are situated some distance away from rivers; their effluents are channeled into rivers such as Ikpoba River [20]; and Eruvbi stream [14]. One of these industries is a brewery industry and the effluent from its operation is conveyed over a distance by an underground tunnel into Ikpoba River. These effluents which are rich in organic and inorganic substances are capable of producing adverse effects on the physical, chemical and biotic components of the environment and either directly or indirectly on human health [16] [22]. Ekhaise and Anyasi [8] carried out a study on the effect of brewery effluents on the physical and chemical characteristics of Ikpoba River. From their studies, stated that there was an observed increase in most of the physical and chemical parameters studied with slight variations in the temperature and pH, with the highest values recorded at the effluent discharge points. Imoobe and Okoye [14] also reported on the effect of the effluent produced by a soft drink industry on Eruvbi stream which is a tributary of Ikpoba River. From their findings on the physical and chemical analysis, they observed that water temperature, turbidity, alkalinity, hardness, calcium and magnesium were significantly higher at the effluent discharge point than other section of the stream. When values obtained were compared with Federal Environmental Protection Agency (FEPA) limit and WHO guideline values, it was noted that the turbidity in the effluent discharge point and the downstream were found to exceed the minimum allowable limit while dissolved oxygen level at all stations were below minimum allowable limit for aquatic life [28].The fundamental importance of freshwater for life on the earth needs little justification. It is a limiting factor for industrial development and irrigation of crops [32] [31]. Water is a fundamental force in ecological life-support systems on which sustainable social and economic development depend [30] [34]. Studies have shown that water quality in aquatic ecosystems has a strong ecological impact [33] [35]. Freshwater is not only essential to life, but also clearly a relatively scarce resource and this is likely to become more so with the impacts of climate change. The costs of correcting degraded water and dealing with unforeseen conflicts over water shortages may be very high for future generations to come. An estimated 25% of the world’s food market is driven by water scarcity [36]. There has been concern in Benin City about the health of Ikpoba River due to effluents being discharged into the river by a Benin-based brewery company which discharges its waste effluent. Fears have been raised that due to the discharges of this effluent, the river could be polluted excessively. Hence the need to assess the state and quality of Ikpoba River water becomes imperative given that the water is used for domestic use, irrigation and livestock rearing. The river is of importance not only to people who live, fish and farm in Benin City but also to citizens in faraway towns, who consume fish from the river.
2. Materials and Methods
2.1. Study Area
- Ikpoba River, a fourth order stream, is located in Benin City, Edo State in South South Nigeria (Lat 6.5°N, Long 5.8°E). Its headwater originates from North West of Benin City and flows north to south through the city (Benka-Coker and Ojior, 1995). The river flows through a dense rain forest where the allochtonous input of organic matter from the surrounding vegetation is derived through run-off from the surface of the soil. A rhythm of rainfall occurs in conjunction with the movement of the South West monsoon wind across the Atlantic Ocean and the timing of these movements varies from year to year. Typically, the regions have the characteristic features of the humid tropical wet and dry climate governed primarily by the rainfall. The vegetation of Ikpoba River consists of secondary rainforest, which has been greatly subjected to deforestation and other human activities. The dominant vegetation comprises of rubber palm, bamboo (Bambusa bambusa), and palm trees with epiphytic ferns growing on it.
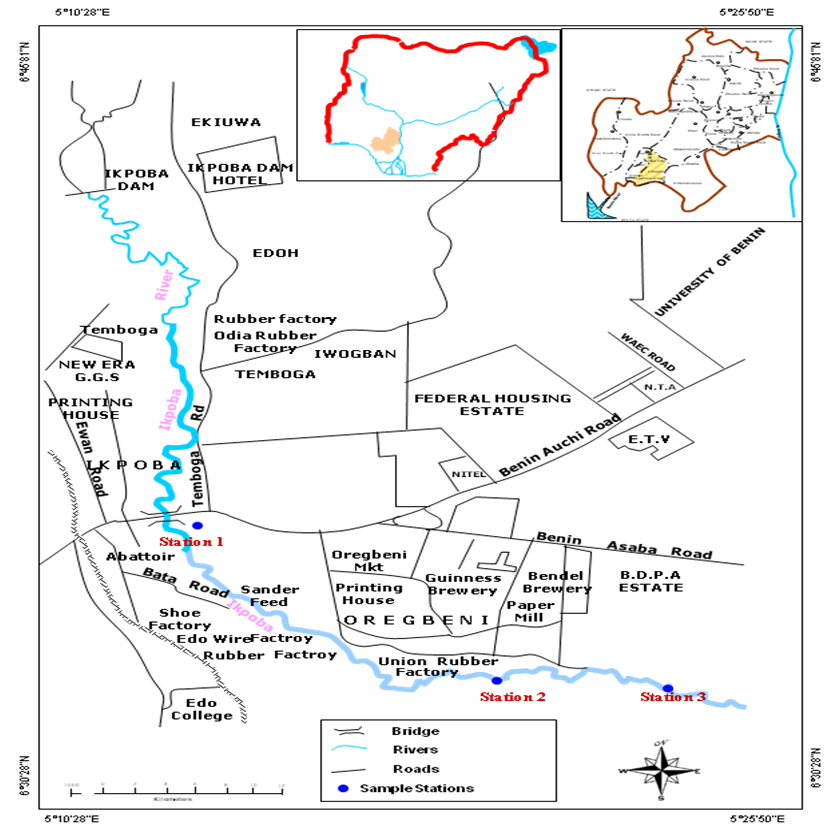 | Figure 1. Map of the study area (Map of Nigeria and Edo State as inset) |
2.2. Sample Stations
- Three sampling stations were selected for this study. Station 1 is the upstream section of the river, the point, just after the bridge. Water velocity is high and depth is minimal when compared to other stations. At this section the water is clear, and the substratum is silty. Human activities include fishing and domestic activities such as bathing and washing of clothes and cars, mechanic workshops and the activities of the local deity shrine. Station 2, the discharge point was located 400m downstream of station 1. It was the contact point of the brewery effluent with the river water before it flows down to station 3. The substratum is coarsely sandy with pebbles. The river bank is muddy and has a large deposit of spent grain which has accumulated over time as a result of the effluent flow. The water is highly turbid and the odour is from this section of the river is stinking. The activities in this region include fishing. Station 3 was located about 100m downstream of station 2. The water is turbid; the water level is higher than station 1 and 2. The activities in this section include distillation of palm wine to alcoholic beverages, fishing and farming activities.
2.3. Collection of Water Samples
- Monthly sampling of Ikpoba River was carried out in the wet season months of April to September, 2012. Samples were collected between the hours of 0900 and 1200hours on each sampling day. Each time, sampling began in station 1 and terminated in station 3. Acceptable standard methods and instrumentations were followed during sample collection procedures [3]. At each station, the surface water temperature was taken in-situ. Surface water samples for physicochemical analyses were collected into thoroughly cleaned 1liter polyethylene bottles and tightly closed. Each bottle was rinsed with the appropriate sample before the final sample collection. The samples were placed in a cooler box and then taken to the laboratory for analyses. For dissolved oxygen (DO) determinations, separate samples were collected in 300 ml plain glass bottles and the samples fixed using the azide modification of Winkler’s method [3]. Samples for biochemical oxygen demand (BOD) were collected into dark glass bottles for incubation and subsequent DO determination. In the laboratory pH, total dissolved solids, electrical conductivity, turbidity, dissolved oxygen, biochemical oxygen demand (BOD5), chemical oxygen demand (COD), chloride, sodium, potassium, magnesium, sulphate, nitrate, phosphate, lead, iron, zinc, cadmium and copper were determined according to procedures outlined in the Standard Methods for the Examination of Water and Wastewater [3]. pH was measured using a HACH digital meter, total dissolved solid was determined using a TDS meter (Model 4076), electrical conductivity was measured using Cybersan 510 conductivity meter, while turbidity was determined using a DR/2000 HACH spectrophotometer. Titrimetric method was used in the determination of chemical oxygen demand (COD), chloride, sodium, potassium, and magnesium. Sulphate, nitrate and phosphate were determined spectrophotometrically at 380nm, 470nm and 680nm respectively. Heavy metal such as lead, iron, zinc, cadmium and copper in the water sample was determined using Atomic Absorption Spectrophotometer (Buck Scientific Model-210) at 217.0nm, 248.0nm, 213.9nm, 228.8nm and 324.8nm respectively.
2.4. Statistical Analysis
- Data was analysed using Statistical Package for Social Sciences (SPSS 16.0) to test for significant differences within the physicochemical parameters using One-way analysis of variance (ANOVA). Where significant values (p < 0.05) were obtained, ‘A posteriori’ Duncan Multiple Range Test was subsequently applied to all pairs of means to detect the location of difference.
3. Results
- A summary of the results of physico-chemical analyses are presented in Table 1. These values were placed alongside WHO guideline values [28]. The monthly variations across the stations are also shown in Table 2. The pH was slightly acidic in all stations, although within WHO limit. It was however not significantly different (P>0.05). Monthly variation indicated that pH in all station increased in value from April to September. Surface water temperature fluctuated between 26 and 39°C during the entire period of study. Mean temperature in Station 2 was significantly higher (P<0.05) than temperature in stations 1 and 3. However, temperature in all stations was above WHO limit. There was no temporal variation in the mean water temperature of the study area. Mean TDS concentration was highest in station 2, and was significantly different (P<0.05) from stations 1 and 3. There was also significant temporal variation in the mean TDS of the study area. Water conductivity was significantly higher (P<0.05) in station 2 than in station and 3. In station 2, conductivity decreased in value with the highest value recorded in September and lowest in April. Turbidity measured in NTU was not significantly higher (P>0.05) in station 2 than stations 1 and 3. Mean values recorded in all stations were beyond WHO standard (5NTU). The mean DO was generally low in all stations, with lowest value recorded in station 2. However, there was no significant difference (P>0.05) in the DO concentration among the stations. BOD was lower in station 1 and 3. There was also no significant difference (P>0.05) in the mean BOD values across the stations. Mean COD value in station 2 was not significantly (P>0.05) higher than in station 1 and 3. Monthly variation showed that COD decreased abruptly from April to June in all station, then slightly increased in July and remained fairly constant to September. Chloride concentration was significantly (P<0.05) higher in station 2 than other stations, although within WHO limit. The concentration of sodium ranged from 0.91 to 28.46mg/l during the entire period of study. However, mean value in station 2 was significantly higher than station 1 and 3. Mean potassium and magnesium value was however not significantly higher in station 2 than other stations. The mean sulphate value was lowest in station 1 and highest in station 2. Station 2 had significantly higher (P<0.05) values than stations 1 and 3. Nitrate concentration was significantly higher (P< 0.05) at station 2 than other stations. The concentration at station 2 exceeded WHO limit. Phosphate was also higher at station 2 than other stations, although not significantly different. The mean value of lead in station 2 was above limit; and was significantly higher (P<0.05) at station 2 than in stations 1 and 3. Iron concentration was higher at stations 2 and 1 than in station 3. It ranged from 6.67 to 93.33 mg/l, and was below WHO limit in all stations. Zinc and copper ranged from 0.01 to 0.97mg/l and 0.00 to 2.75mg/l respectively throughout the period of study. Zinc was significantly different at station 2 than other stations, while copper was not significantly different. Mean cadmium value at station 2 was above recommended limit. It however ranged from 0.00 to 0.92mg/l in all stations. It therefore varied significantly across the stations. The spatial variations in all the physicochemical parameters investigated shown in Tables 1 and 2 indicate that all parameters except pH and biochemical oxygen demand (BOD5) were clearly higher in values at station 2, the point of effluent discharge into the stream.The relationship between the physico-chemical parameters was examined using the Pearson’s correlation analysis and the results are shown in the form of a correlation matrix (Table 3). pH inversely exhibited a significant correlation only with turbidity, sodium, potassium, sulphate, lead and cadmium (r = -0.537, -0.589, -0.527, -0.515, -0.568 and -0.503 respectively). Temperature correlated significantly only with COD (r = 0.514) and potassium (r = 0.500). TDS indicated a significant correlation with chloride (r = 0.514), sulphate (r = 0.485) and zinc (r = 0.562). EC was directly correlated with sodium (0.571), magnesium (0.553), nitrate (0.512), phosphate (0.573) and iron (0.486). Turbidity revealed a significant correlation with BOD and COD (r = 0.483 and 0.563 respectively). BOD correlated significantly with COD (r = 0.587), nitrate (r = 0.527), phosphate (r = 0.503), iron (r = 0.499) and copper (r = 0.586). COD directly correlated with sodium and potassium (r = 0.480, 0.560 respectively). Chloride correlated with zinc (r = 0.558). Potassium correlated with iron and copper (r = 0.501, 0.545 respectively). While magnesium correlated with sulphate (r = 0.562).
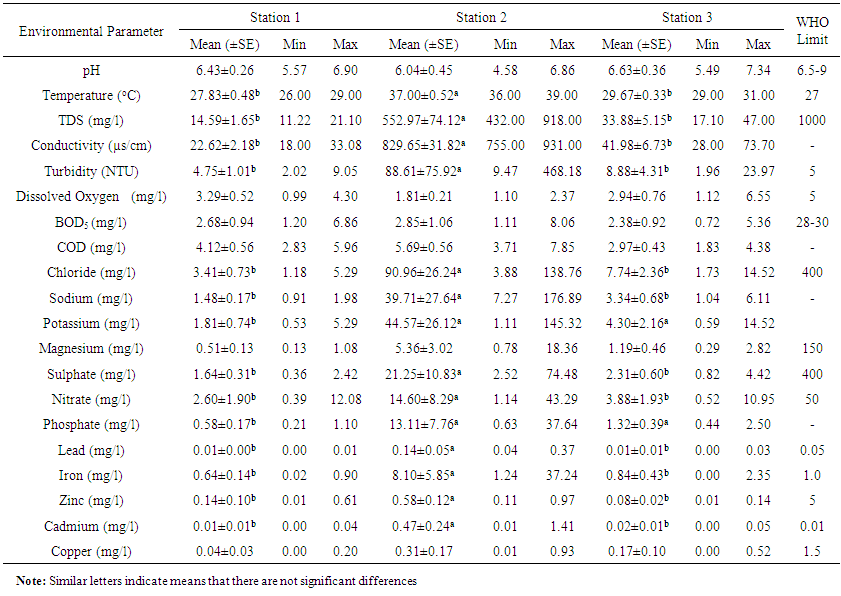 | Table 1. Some physical and chemical conditions of Ikpoba River water at three sampling stations from April to September, 2012 |
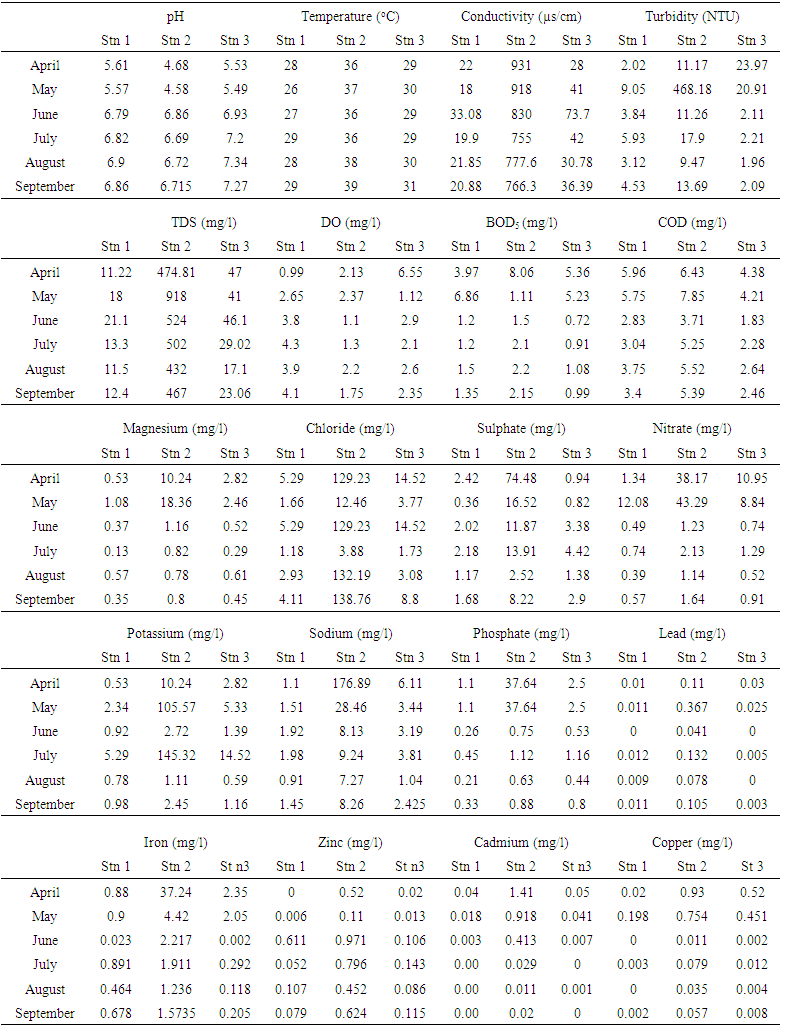 | Table 2. Temporal and spatial variation of some physical and chemical characteristics of Ikpoba river from April to September, 2012 |
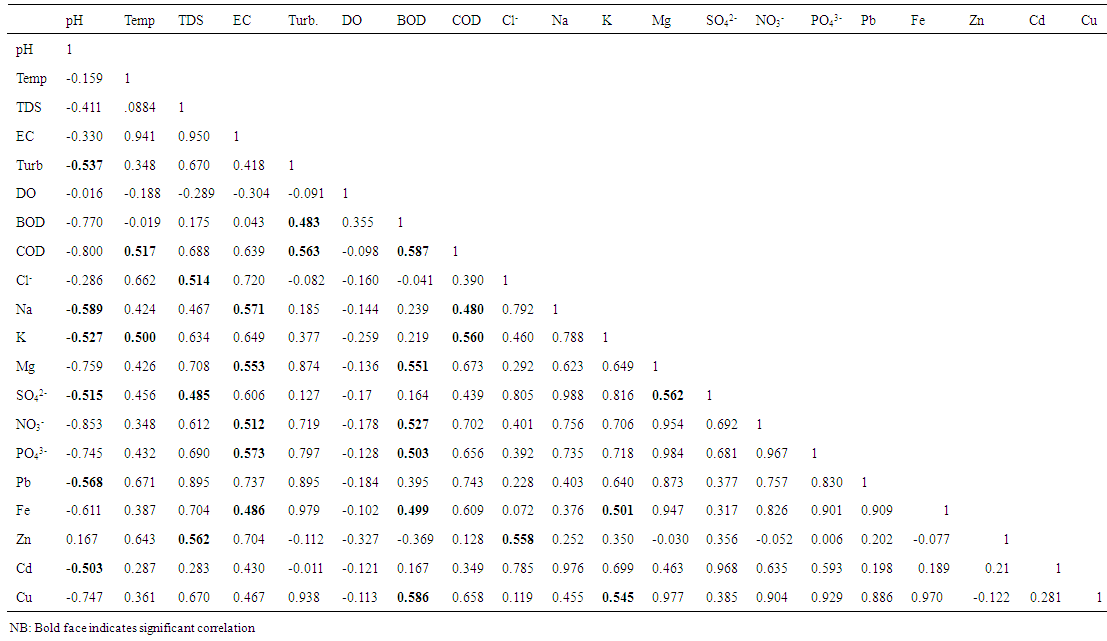 | Table 3. Correlation matrix for physical and chemical parameters of Ikpoba river from April to September, 2012 |
4. Discussion
- Temperature is important in aquatic systems. Water temperature is naturally influenced by substrate composition, turbidity, vegetation cover, run-off, inflows and heat exchange with the air [4] [12] [26]. The high temperature value reported from station 2 can be attributed to firstly, the discharge of warm effluent and steam condensates into the receiving water and second, the increased organic load, that might have caused an increase in microbial activities hence absorption of heat. This is in agreement with the findings of Imoobe and Okoye [14] and Ekhaise and Anyasi [8] for effluent from a soft drink factory and brewery effluent discharge at Eruvbi stream and Ikpoba River respectively. High temperature increases speed of metabolic reactions as well as reduce the solubility of dissolved oxygen (DO) in the water column which is detrimental to aquatic life [18]. pH is a measure of the acidity or alkalinity of a substance and is one of the stable measurements. pH is a simple parameter but extremely important, since most of the chemical reactions in aquatic environment are controlled by changes in its value [10]. The pH obtained from this study was well within acceptable limit [29]. Similar range of 6.8 - 7.2 was recorded by Ovie and Adeniji [23] for Shiroro Lake while Ovie [22] recorded between 6.9 and 7.6 for Jebba Lake. Conductivity of water is the numerical expression of the ability of an aqueous solution to carry electric current. This ability is a function of the presence of ions` (cations and anions), their total concentration, mobility, valency and temperature measurement. While distilled water is almost an insulator, salt water is a very efficient electrical conductor. In this study, high value of conductivity though much below WHO minimum allowable limit (1000mg/L) for aquatic life, was recorded at the effluent discharged point. This may be attributed to the effluent inflow containing high concentrations of cations such as sodium and magnesium and anions such as chloride which were also high at the effluent discharge point. Again, pearson correlation showed that conductivity correlate significantly with sodium, magnesium, nitrate, phosphate and iron. Similar trend has also been reported by Imoobe and Okoye [14] when they reported higher conductivity value at the effluent discharge point of a river receiving effluent from a soft drink factory. Higher mean value of total dissolved solids was recorded in station 2 (552.97mg/l), though this was within the permissible levels of drinking water, it indicated high levels of dissolved ions in the effluent as corroborated by the higher conductivity level in station 2. Alabaster and Lloyd (1980) reported that excessive concentration of suspended and dissolved solids might be harmful to aquatic life, because they decrease water quality, inhibit photosynthetic processes and eventually lead to increase of bottom sediments and decrease of water depth [19].Turbidity refers to water clarity. The greater the amount of suspended solids in the water, the murkier it appears, and the higher the measured turbidity. This indicates an increase in the concentration of suspended matters in the water as a result of the nature of effluent and effluent inflow which subsequently reduced downstream station 3. This is indicative of self-purification by the river [9]. Turbidity of the water increased greatly from 88.61 NTU at the point of entry (station 2) and reduced drastically to 8.88 NTU in station 3 due to downstream dilution. This indicates an increase in the concentration of suspended matters in the water as a result of effluent inflow and the subsequent reduction at the downstream station 3 is indicative of self-purification by the river [9]. The discharge of effluents directly into station 2 significantly increased turbidity and reduced the dissolved oxygen level. This is in agreement with the studies of Chikere and Okpokwasili [6] that reported that exogenous substances of organic origin are known to increase water colour, turbidity, and suspended and dissolved solids. The dissolved oxygen concentration is a function of temperature, pressure, salinity and biological activity in the water body. Tropical aquatic ecosystem should have a dissolved oxygen concentration of at least 5mg/l in order to support diversified biota, including fish [15]. The study area was not well aerated, as the level was below WHO [29] standard value of 5mg/l necessary for aquatic productivity. The recorded DO mean of 1.81mg/l (station 2) could be attributed to the presence of high concentrations of degradable organic and inorganic matters which resulted in a tendency to be more oxygen demanding, making oxygen less available to the desirable organisms including fishes. This is in agreement with the observations made by Ogbeibu and Edutie [20] who reported a dissolved oxygen range of 1.84 – 5.22mg/l in Ikpoba River. Again, similar range (4.5 to 6.4mg/l) has been reported by Chikere and Okpokwasili [6] for Eleme River in Niger Delta receiving petrochemical effluent. Imoobe and Okoye [14] has also reported a drop in dissolved oxygen value at the effluent discharge point along Eruvbi stream in response to effluent from a soft drink industry in Benin city.BOD and COD represent the amount of oxygen required for the biological and chemical decomposition of either organic or inorganic matter respectively under aerobic conditions at a standardized temperature in surface water (e.g. lakes and rivers) or leachate [29]. High BOD and COD values was recorded in station 2, the effluent discharge point; though below the WHO [29] standard value of 30 mg/l could be attributed to partial or non-treatment of the effluents by the industry before releasing them into waste receiving streams. It is an indication of a high level of pollution, which could result in high biodegradation activity by microbes. High BOD and COD has undesirable consequence on aquatic life such as leading to the production of ammonia and hydrogen sulphide [5] which affect fish negatively in various ways. The biodegradation of organic and inorganic materials exerts oxygen tension in the water and increases the biochemical oxygen demand. This implies that it is dangerous to discharge the effluent directly into water without aeration, as this would deplete the water of dissolved oxygen that is needed by aquatic animals for respiration. This is because high BOD and COD leads to less dissolved oxygen, which is detrimental to aquatic lives [15].Nitrates and phosphates have often been cited as the limiting nutrients in aquatic systems and as indices of eutrophication in lakes, rivers and reservoir [27] [25] [22]. They play vital role in the biological metabolism of aquatic organisms (notably phytoplankton and macrophytes). Concentration of nitrate production in water at any given time is a product of balance between nitrate productions through the activities of nitrifying bacteria and nitrate destruction by autotrophic assimilation and/or bacteria denitrification [11]. In this study, nitrate and phosphate were significantly higher in the effluent discharged point, although below WHO [29] limit. This may be as a result of the brewery effluent discharge at this station. However, the high nitrate concentration reported at the effluent discharge point is in agreement with the findings of Imoobe and Okoye [14] who also reported increased nitrate concentration in Eruvbi stream as a result of effluent from soft drink factory. Lead, iron, zinc, cadmium and copper are toxic heavy metals in the environment when present beyond required levels. However, the sources of these metals generally in the environment are from metal mining activities, metal smelting activities, other metal-using industries, waste disposal, corrosion of metals in use, agriculture and forestry, and fossil fuel combustion. They can bind with important enzymes and inactivate them. They can also displace biologically important metals, such as Calcium, Zinc and Magnesium, interfering with a variety of the body’s chemical reactions [1]. In this study, all he heavy metals studied were higher in station 2, while lead, iron and cadmium were found to be above the WHO [29] acceptable limits. These heavy may be as a result of the effluent discharged from the brewery factory. Similarly Imoobe and Okoye [14] have also reported higher level of heavy metals in Eruvbi stream as a result of effluent from a soft drink factory in Benin City. These metals are subject to bioaccumulation and may cause risk to human health when transferred to the food chain [7]. Exposure of heavy metals may cause blood and bone disorders, kidney damage, decreased mental capacity and neurological damage [17]. The increased levels of electrical conductivity, nitrate, and cations are products of decomposition and mineralization of organic materials, at the discharge point (station 2) and downstream station 3. The biological significance of this is the disruption of the delicate ecological balance of the ecosystem, a reduction in population of organisms, and a subsequent loss of the already depleted biodiversity of the stream (Imoobe and Ohiozebau, 2009). The interesting part of this work shows that due to the power of nature, most of these impacted parameters appear to have easily recovered to their original state downstream.
5. Conclusions
- The changes observed in some of the physical and chemical properties of Ikpoba River, notably increased temperature, turbidity, nitrate, lead, iron and cadmium at Stations 2 has shown that the quality of receiving water is significantly influenced by the effluents discharged into the River. However, specific quality criteria must be met by the breweries especially before the discharge; as the river serves as a source of domestic water supply for the local communities; hence the following recommendations;1. Strict adherence to stipulated guidelines for effluent generated by industries coupled with adequate and regular monitoring of effluent discharge into the aquatic environment should be undertaken to halt further deterioration of the environment that might jeopardize human health.2. Automated measuring and monitoring equipment should be installed to check discharge parameters against stipulated standards for drinking water, aquatic life and other uses.
ACKNOWLEDGEMENTS
- We are grateful to Professor C.G. Oronsaye of the Department of Animal and Environmental Biology, University of Benin for guiding us with this research; and also Mr Bayo Lawal of Tudaka Enviromental consultancy Laboratory for assisting us with the laboratory analysis during this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML