-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2015; 5(6): 173-181
doi:10.5923/j.re.20150506.01

Ammonium Thiosulphate Assisted Phytoextraction of Mercury and Arsenic in Multi-Polluted Industrial Soil
Francesca Pedron1, Gianniantonio Petruzzelli1, Irene Rosellini1, Meri Barbafieri1, Elisabetta Franchi2, Roberto Bagatin2
1Institute of Ecosystem Study, National Council of Research, Pisa, Italy
2Eni S.p.A, Renewable Energy & Environmental Laboratories, S.Donato Milanese (MI), Italy
Correspondence to: Francesca Pedron, Institute of Ecosystem Study, National Council of Research, Pisa, Italy.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The possibility of using ammonium thiosulphate in assisted phytoextraction was evaluated on a greenhouse scale (mesocosm) for the simultaneous removal of mercury and arsenic from multi-polluted industrial soil. The addition of thiosulphate to the soil greatly promoted the uptake and translocation of both contaminants in the aerial parts of Brassica juncea and Lupinus albus. Thiosulphate showed great potential since it is a common fertilizer used to promote plant growth and is able to promote plant uptake of both Hg and As. Hg concentration in the aerial part of the plants reached 867 mg kg-1 in B. juncea and 114 mg kg-1 in L. albus. In the aerial parts, As concentration was about 9 mg kg-1 in B. juncea and 20 mg kg-1 in L. albus. This thus increases the applicability of phytoextraction in terms of cost and time especially if the remedial targets are based on bioavailable metal concentrations.
Keywords: Thiosulphate, Phytoextraction, Mercury, Arsenic, Greenhouse experiment, Multi-Contaminated soil, Brassica juncea, Lupinus albus, Bioavailability, Repeated growing cycles
Cite this paper: Francesca Pedron, Gianniantonio Petruzzelli, Irene Rosellini, Meri Barbafieri, Elisabetta Franchi, Roberto Bagatin, Ammonium Thiosulphate Assisted Phytoextraction of Mercury and Arsenic in Multi-Polluted Industrial Soil, Resources and Environment, Vol. 5 No. 6, 2015, pp. 173-181. doi: 10.5923/j.re.20150506.01.
Article Outline
1. Introduction
- Mercury and arsenic are widely distributed throughout the environment in the air, water and soil. Both contaminants are present in the atmosphere from volcanoes activity and wildfires. As trace elements, they are naturally present in soils due to their geological origin. Soil weathering can release As and Hg compounds which may be dispersed by wind and rainfall water. However, mercury and arsenic constitute one of the largest environmental problems in former industrial sites, due to the very high concentrations in the soil in comparison to background values [1-3]. Depending on their speciation, which influences their mobility and bioavailability in soil, these two contaminants are particularly dangerous for human health through the direct inhalation of contaminated dust and hand-to-mouth ingestion. Arsenic and mercury are also some of most hazardous contaminants in drinkable and groundwater throughout the world since they can be assimilated and bioaccumulated with occasional biomagnification [4]. Exposure to mercury represents a great hazard for human health, since Hg has no biological function. The most toxic form of Hg, methylmercury, is not present in well-aerated soil as it is mainly produced in aquatic environments [1]. In the soil, Hg can be involved in adsorption and release from solid phases, complexation with organic and inorganic ligands, oxidation-reduction, and methylation [5]. Depending on the redox conditions, Hg can exist in soil as Hg0, Hg2+, Hg22+. Mercury can be present in many organic and inorganic complexed forms depending on pH, ionic strength, and dissolved organic matter in soil [6]. Many compounds of Hg with sulphide are highly stable and insoluble, while inorganic ligands such as Cl− ions promote the release of Hg in the soil solution due to the formation of soluble stable complexes [7]. Long-term exposure to arsenic from the food chain and drinking-water can cause many significant health problems with the development of neurotoxicity and cardiovascular diseases [8-9]. Arsenic and arsenic compounds have also been classified as carcinogenic to humans [10]. Arsenic is present in soil mainly in inorganic forms, the two most commonly found species are As(III) and As(V). In oxidizing conditions, arsenate As(V) species is the most stable, forming strong inner-sphere complexes with the oxides of iron, manganese and aluminium and partly with clay minerals [11-12]. Arsenic interactions with soil organic matter are particularly effective at positively charged sites where it can form both “inner-sphere” and "outer-sphere" complexes [13].In alkaline conditions, As is only slightly involved in adsorption to soil components, while with decreasing pH values the adsorption and precipitation of this element increase thus reducing the amount in soil solution [14].Soil properties that determine Hg and As retention by mineral and organic surfaces are mainly pH, oxides, hydroxides and dissolved ions. Clayey and organic soils have the greatest ability to adsorb these elements. If soils are neutral to alkaline, Hg shows stronger adsorption to mineral components, while As is mainly present in the soil liquid phase. A decrease in pH reduces the adsorption processes of Hg [7], but increases As adsorption and precipitation [12]. The different chemical behaviour of the two metals makes the remediation of soils contaminated from both the elements particularly difficult. However, the presence of more than one metal or metalloid in soil is widespread in contaminated sites. Often in these cases excavation and landfilling, soil washing and stabilization / inertization are the most used solutions for remediation.Among the alternative approaches based on sustainability and the conservation of natural resources, phytoextraction, the use of plants to extract inorganic pollutants from soil, may be a viable solution. Phytoremediation has been intensively investigated due to its environmental friendly approach to remediation, which favours greater social acceptance [15]. Phytoremediation was originally based on the use of metal hyperaccumulator plants, which are characterized by the ability to accumulate high amounts of heavy metals or metalloids [16]. However, the use of hyperaccumulators is often hindered by their reduced production of biomass and by the ability to accumulate only one specific element, which makes them impractical in multi-contaminated soil. As an alternative “assisted phytoextraction” is commonly used. In this technology, high biomass plant species are used together with the modification of soil properties by chemical additives, which increase the bioavailability of the contaminants. Many additives, in particular chelating agents such as ethylenediaminetetra-acetic acid (EDTA), have been commonly used [17-18]. However, these agents persist in the soil with residual toxic effects and there is a risk of metal leaching into the groundwater. New mobilizing agents, which are under study, have no adverse effects on the environment while promoting the bioavailability of contaminants. Among these, thiosulphate appears particularly attractive for mercury remediation since it is a common fertilizer used to promote plant growth and form stable soluble complexes with mercury in soil [19-20-21], thus promoting the uptake of the metal by growing plants. While thiosulphate has been successfully used in many contaminated soils to increase mercury phytoextraction, recent research has shown that thiosulphate competes with arsenate ions for sorption on the same surface of oxides. This competition may, in turn, increase the release of arsenic in the liquid phase of soil and its bioavailability to plant uptake [22]. These results suggested that thiosulphate could be used also to phytoextract arsenic. This solution would be of considerable interest in soils contaminated with both elements.The aim of this paper was to evaluate the applicability of phytoextraction technology to reduce the bioavailable fraction of Hg and As from a soil contaminated by the two elements using only one additive: ammonium thiosulphate. Greenhouse experiments were carried out to compare also the efficiency of thiosulphate with phosphate, which is the typical mobilizing agent for arsenic in assisted phytoextraction.
2. Materials and Methods
2.1. Soil Characterization
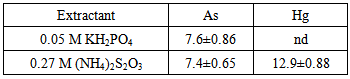
- The soil used in this study was sampled from a former industrial site, where various industrial activities had been carried out. As and Hg contamination was discovered with mean values of 37.6 and 65.8 mg kg-1, respectively. Soil samples were air dried and sieved with a 2 mm sieve for soil analysis. The following parameters were determined: pH, cation exchange capacity (CEC), texture (sand silt and clay) and organic matter [23]. Readily bioavailable mercury and arsenic in the soil solution were determined by H2O extraction by shaking the soil and extractant (ratio 1:5) for 3h, which is often used as the first step in heavy metal sequential extractions [24]. Hg and As were analyzed in the extracts after centrifugation and filtration. Long-term potential bioavailability was determined using 0.27 M ammonium thiosulphate for Hg and 0.05 M potassium biacid phosphate for As, according to the procedure of Enhanced Bioavailable Contaminant Stripping (EBCS) [20-25]. The extractability of As was also tested with 0.27 M ammonium thiosulphate.
2.2. Mesocosm Experiment
- To obtain a representative sample of the field situation, the soil used in these experiments was prepared by eliminating (sieving at 2 cm) the coarser materials, however without sieving to 2 mm. Experiments were carried out in a greenhouse. Mesocosms were polypropylene containers filled with 4 kg of soil. Each pot had a hole in the middle of the base where a plastic tube was inserted to collect the leachate in a plastic bottle [20].The mesocosm experiments lasted 60 days. The plant species selected for the tests, Brassica juncea and Lupinus albus, were considered viable candidates for phytoremediation due to their ability to grow in a Mediterranean climate and their relative tolerance to heavy metal soil contamination [18-20]. A total of 1.0 g per pot of B. juncea seeds or nine seeds per pot of L. albus were used in four replicates for each species. Thirty days after sowing, a solution of ammonium thiosulphate 0.27 M (NH4)2S2O3 (T treatment) or a solution of potassium biacid phosphate 0.05 M KH2PO4 (P treatment) as mobilizing agents for Hg [19-21] and for As [26], respectively. After the first harvest a second growing cycle was performed. On some mesocosms the mobilizing agents were added with the same doses as the first cycle (T/T, P/P), in others no further treatment (nt) was carried out (T/nt, P/nt).Both additives were added to the soil by splitting the total dose into five-day applications to minimize the possible toxic effects on the plant species. Control mesocosms (CT) for each species were run simultaneously in untreated soil. At the end of the growing period, plants were harvested and aerial parts and roots were separated. Vegetal samples were washed with deionized water; the roots were further washed in an ultrasound bath (Branson Sonifier 250 ultrasonic processor; Branson, Danbury, Conn.) for 10 min to eliminate any soil particles from the root surfaces. The dry biomass of shoots and roots was gravimetrically determined after maintaining samples at 40°C until a constant weight was achieved. The dry plant samples were ground and digested with an acid mixture (HNO3+H2O2) for As and Hg analysis.
2.3. Determination of As and Hg
- As concentration in soil, plant samples, and soil extracts was determined by ICP-OES (Varian AX Liberty) with a method for the generation of hydrides [23]. The mercury concentration in the same samples was determined using atomic absorption spectroscopy with an Automatic Mercury Analyzer (AMA 254, FKV, Bergamo, Italy), according to the SW-846 method 7473 (27 U.S. EPA, 1998).
2.4. Quality Assurance and Quality Control
- Quality assurance and quality control were performed by testing standard solution every 10 samples. Certified reference material (BCR n°141) was used to control the quality of analytical system. The detection limits were 2 µg L-1 for Hg and 0.05 mg L-1 for As, respectively.The recovery of spiked samples ranged from 93 to 101% with a RSD of 1.89 of the mean.
2.5. Statistical Analysis
- Statistical analyse was performed using Statistica version 6.0 (Statsoft, Inc.). Effects of mobilizing agents were analyzed using one-way analysis of variance (ANOVA). Differences among means were compared and a post-hoc analysis of variance was performed using the Tukey Honestly and significant differences considered at p < 0.05.
3. Results and Discussion
3.1. Soil Characteristics
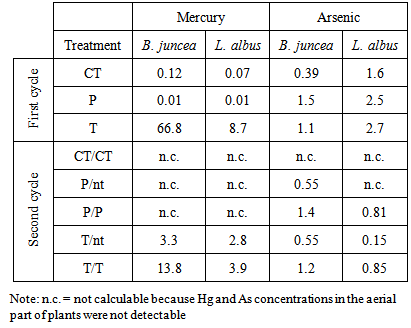
- Soil was characterized by a pH value of 8.06, 1.5% of organic matterand a cation exchange capacity of 15.6 cmol(+) kg-1. The soil can be defined as sandy according to the texture values: 78.9% sand, 13.1% silt and 8.0% clay. To evaluate the potential efficiency of thiosulphate as a solubilizing agent, Hg and As extractability was determined by 0.27 M (NH4)2S2O3 extraction. As extractability was also determined by 0.05 M KH2PO4 the typical mobilizing agent for arsenic. Results showed that (NH4)2S2O3 was able to extract the same amounts of As as those extracted by phosphate (Table 1). These results highlighted the effectiveness of thiosulphate as single mobilizing agent for assisted phytoextraction in this contaminated soil.
|
3.2. Biomass Production and Metal Uptake
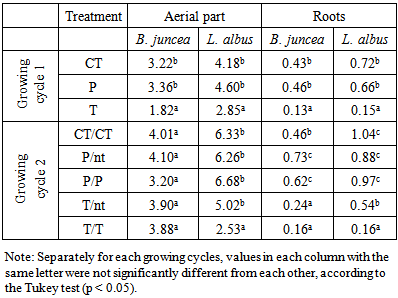
- The biomass data, expressed as g dry weight, are reported in Table 2, in which for the first growing cycle the controls are indicated by CT, while P and T are the treated mesocosms with phosphate and thiosulphate, respectively. For the second growing cycle the letters after the slash indicates the presence (P/P or T/T) or not (P/nt or T/nt) of a second treatment. Control pots were obviously not treated.
|
|
|
3.3. Total Accumulation
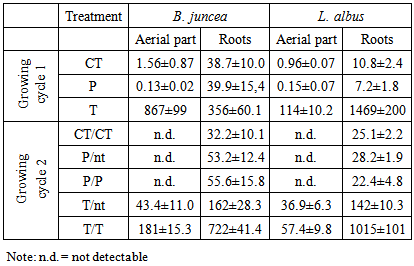
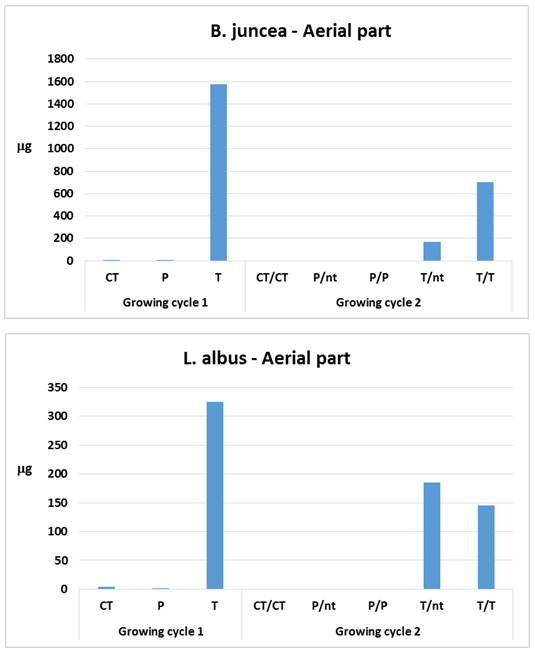
- The product of As and Hg concentration values in shoots and the corresponding biomass, expressed as "total accumulation" [29], provides an estimate of the amount of contaminants removed from the polluted soil and therefore of the efficiency of phytoextraction. The data obtained using the mean values in the calculation are reported in Figure 1 for mercury and Figure 2 for arsenic.
 | Figure 1. Hg total uptake 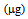 of the two plant species in mesocosm experiment of the two plant species in mesocosm experiment |
 | Figure 2. As total uptake 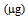 of the two plant species in mesocosm experiment of the two plant species in mesocosm experiment |
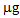 in the first growing cycle and around 700
in the first growing cycle and around 700 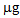 in the second after a further treatment with thiosulphate. In the controls and in the pots treated with phosphate, these values were negligible. With regard to L. albus, the amount of Hg accumulated in the plants was lower than that found for B. juncea, but the trend was similar. In the first growing cycle, after the T treatment the value obtained was 325
in the second after a further treatment with thiosulphate. In the controls and in the pots treated with phosphate, these values were negligible. With regard to L. albus, the amount of Hg accumulated in the plants was lower than that found for B. juncea, but the trend was similar. In the first growing cycle, after the T treatment the value obtained was 325 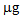 , while after the second cycle, this decreased to about 160
, while after the second cycle, this decreased to about 160 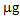 (T/nt and T/T). In the controls and after the P treatment, the accumulation was much lower or even below the detection limit. Regarding the As accumulated in the aerial parts of the plants, both treatments promoted the absorption of the contaminant compared to the controls, thus confirming the mobilizing action on As not only of phosphate, but also of thiosulphate [22]. In the case of B. juncea, in the first growing cycle, plants grown in the control pots showed a mean total accumulation of 10
(T/nt and T/T). In the controls and after the P treatment, the accumulation was much lower or even below the detection limit. Regarding the As accumulated in the aerial parts of the plants, both treatments promoted the absorption of the contaminant compared to the controls, thus confirming the mobilizing action on As not only of phosphate, but also of thiosulphate [22]. In the case of B. juncea, in the first growing cycle, plants grown in the control pots showed a mean total accumulation of 10 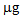 . After treatments with thiosulphate and phosphate this value increased to 15.8 and 39.0
. After treatments with thiosulphate and phosphate this value increased to 15.8 and 39.0 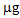 , respectively. In the second growing cycle, the highest total accumulation (36
, respectively. In the second growing cycle, the highest total accumulation (36 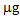 ) was determined in the plants after a repeated treatment (T/T and P/P), while in the P/nt and T/nt pots the value was about 17
) was determined in the plants after a repeated treatment (T/T and P/P), while in the P/nt and T/nt pots the value was about 17 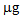 .In the case of L. albus in the first growing cycle, the total accumulation was higher than that obtained for B. juncea and ranged from a minimum of 50.2
.In the case of L. albus in the first growing cycle, the total accumulation was higher than that obtained for B. juncea and ranged from a minimum of 50.2 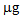 in the controls to a maximum of 86.9
in the controls to a maximum of 86.9 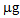 after the P treatment. In the second growing cycle, the maximum accumulation was determined after the repeated treatment with phosphate P/P (42.3
after the P treatment. In the second growing cycle, the maximum accumulation was determined after the repeated treatment with phosphate P/P (42.3 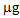 ). A comparison between the two tested plant species should take into account the simultaneous presence of the two contaminants, Hg and As. The greatest amount of Hg was taken up by B. juncea following the T treatment, which also promoted a notable increase in As uptake compared to the controls. L. albus accumulated higher amounts of As than B. juncea but much lower amounts of Hg. Thus, the use of B. juncea in combination with T treatment appears to be the best available choice in this contaminated soil. The data obtained with the use of thiosulphate should be considered very positively since they show that only one mobilizing agent was able to simultaneously increase the absorption of Hg and As by plants. The addition of thiosulphate has often led to very positive results for Hg phytoextraction in different contaminated soils [19-20-21]. In contrast, the effect of thiosulphate on the bioavailability of As has not been widely investigated and is of great interest. A recent study on the competitive adsorption between arsenate and thiosulphate ions, highlighted the great influence of thiosulphate on As adsorption by hematite [22]. The competition can be ascribed to an inner-sphere complexation, with the possible formation of monodentate non protonated surface complexes similar to those formed by phosphate in the adsorption on hematite [30]. Thiosulphate ion can be considered as a sulphate ion and a sulphide ion linked by a double bond [31]. In soil, thiosulphate decomposes into sulphur and sulphate. Sulphur can give rise to precipitates while sulphate remains in solution according to the following reaction: thiosulphate, tetrathionate, sulphite, sulphate.
). A comparison between the two tested plant species should take into account the simultaneous presence of the two contaminants, Hg and As. The greatest amount of Hg was taken up by B. juncea following the T treatment, which also promoted a notable increase in As uptake compared to the controls. L. albus accumulated higher amounts of As than B. juncea but much lower amounts of Hg. Thus, the use of B. juncea in combination with T treatment appears to be the best available choice in this contaminated soil. The data obtained with the use of thiosulphate should be considered very positively since they show that only one mobilizing agent was able to simultaneously increase the absorption of Hg and As by plants. The addition of thiosulphate has often led to very positive results for Hg phytoextraction in different contaminated soils [19-20-21]. In contrast, the effect of thiosulphate on the bioavailability of As has not been widely investigated and is of great interest. A recent study on the competitive adsorption between arsenate and thiosulphate ions, highlighted the great influence of thiosulphate on As adsorption by hematite [22]. The competition can be ascribed to an inner-sphere complexation, with the possible formation of monodentate non protonated surface complexes similar to those formed by phosphate in the adsorption on hematite [30]. Thiosulphate ion can be considered as a sulphate ion and a sulphide ion linked by a double bond [31]. In soil, thiosulphate decomposes into sulphur and sulphate. Sulphur can give rise to precipitates while sulphate remains in solution according to the following reaction: thiosulphate, tetrathionate, sulphite, sulphate. 
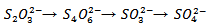 The reaction is either abiotic or biotic with kinetics of oxidation depending on the chemical and biological characteristics of the soil In the presence of plants, which increase the microbiological activity, the transformation of thiosulphate may follow a different pattern [32] without producing tetrathionate as an intermediate:
The reaction is either abiotic or biotic with kinetics of oxidation depending on the chemical and biological characteristics of the soil In the presence of plants, which increase the microbiological activity, the transformation of thiosulphate may follow a different pattern [32] without producing tetrathionate as an intermediate: 
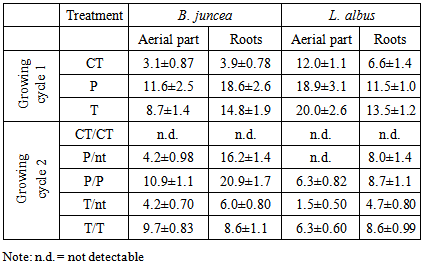
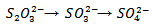 Thus, thiosulphate may substitute arsenate on iron oxides by the sulphate part of the molecule, with oxygen atoms bonding to the surface Fe ions. This competition is essential in determining the As available for plant uptake, since arsenate in soil is mainly retained by Fe oxides. Thiosulphate ions competing for sorption on the same surface may significantly reduce the arsenate adsorption and enhance the mobility of arsenic in soil, similarly to the influence of phosphate on arsenate. In alkaline soil, the competition between the two anions is very high [33] thus, we can assume that the excess of sulphate ions, deriving from the addition of thiosulphate, released arsenate ions in the soil solution, which were thus bioavailable for plant uptake. However, it is also important to consider the interactions between sulphur and arsenic. Sulphur can promote arsenic absorption and transport to the aerial parts of the plants, since sulphur plays an anti-stress role in reducing the toxicity of arsenic [34]. Thiosulphate can thus act as a detoxifying agent by stimulating the defence system of plants while increasing the efficiency of phytoremediation due to competition between arsenate and sulphate ions for the same sites on the soil surfaces. One of the main drawbacks of assisted phytoextraction is the potential leaching of the contaminants solubilized by the additives used, however in our experimental conditions, no arsenic and mercury were found in the small amounts of leachate produced following the irrigation necessary for the plant growth. However, as a precautionary principle, we determined the Hg and As water extractable in soil after the first and the second growing cycles. We can suppose that the addition of thiosulphate mobilizes contaminants and the plants are able to uptake only a part of this amount.Following the T treatment, the soluble amount of Hg was around 2.5 mg kg-1 after the first growing cycle. Without further T addition, after the second growing cycle, the values decreased to about 0.7 mg kg-1 and 1.3 mg kg-1 for pots planted with B. juncea and L. albus, respectively. In the mesocosms treated twice with thiosulphate (T/T) the water soluble Hg amount in soil was about 1.5 mg kg-1 for all the pots. The results showed that a certain amount of mercury remained in the soil, bioavailable for further growing cycles essentially in the rhizosphere. In fact, water extractable Hg decreased after the second growth. These results are in accordance with previous findings [21] that reported an increase in soluble mercury in the rhizosphere following thiosulphate addition did not correspond to an increase in the bulk soil. The results were explained as the consequence of mercury complex decomposition with the production of sulphate ions and subsequently a decrease in mobile mercury species [21].Similar results were obtained for As. Following the T treatment, the water extractable As after the first growing cycle accounted for 1.4 and 0.91 mg kg-1 for B. juncea and L. albus mesocosms, respectively. After the second growing cycle without any further T addition (T/nt), these extractable amounts decreased slightly to 0.80 and 0.65 mg kg-1 in the pots planted with B. juncea and L. albus, respectively. The amounts increased slightly to 1.30 and 0.80 mg kg-1 when the T treatment was repeated (T/T) in the pots planted with B. juncea and L. albus, respectively. This procedure, which involves H2O extraction at the end of the growing cycle, may provide a linkage between plant uptake and the residual bioavailable fraction of the two metals. This is useful when planning other growing cycles aimed at reducing and possibly eliminating the amounts of bioavailable Hg and As which are the most dangerous for humans and the environment [25]. Unlike the use of chelating agents such as EDTA, it is not necessary to apply large amounts of thiosulphate to counteract the co-solubilization of Ca due to the low solubility of CaSO4. The risk of additive leaching is negligible, in contrast to the case of poorly biodegradable chelating agents, such as EDTA. Adverse effects on soil quality can be ruled out since the decomposition of thiosulphate produced sulphur and sulphate, which are essential for fertility.The efficiency of phytoextraction in cleaning up metal-contaminated soils has been questioned due to the long time required to reach the remediation target based on total metal concentration. However, not all the total metals in soils may be involved in environmental processes. There is growing interest in using phytoextraction to reduce only the bioavailable fractions, which are the most dangerous to the environment and human health. The clean-up time can thus be substantially shortened and the imbalance between the potential of phytoextraction and full-scale applicability can be overcome [20].The potential use of plants for phytoextraction can be derived from the ratio between the concentration of Hg and As in the shoots and the total concentration of the two elements in soil. This relationship is often called the phytoextraction coefficient (PEC) or translocation factor (TF). If the aim of phytoextraction is to remove the bioavailable fraction, this coefficient is calculated as the ratio between the concentration of the metal in the aerial parts of the plants and the bioavailable concentration in the soil. This ratio has been defined bioavailability factor (BF) [35].Table 5 summarizes the BF data considering the ratio between the concentration in the plant shoots following each treatment and the potential bioavailability of the contaminants determined by the use of 0.27 M thiosulphate. As previously reported, the As extractable by 0.27 M thiosulphate was the same as that extracted by 0.05 M phosphate.
Thus, thiosulphate may substitute arsenate on iron oxides by the sulphate part of the molecule, with oxygen atoms bonding to the surface Fe ions. This competition is essential in determining the As available for plant uptake, since arsenate in soil is mainly retained by Fe oxides. Thiosulphate ions competing for sorption on the same surface may significantly reduce the arsenate adsorption and enhance the mobility of arsenic in soil, similarly to the influence of phosphate on arsenate. In alkaline soil, the competition between the two anions is very high [33] thus, we can assume that the excess of sulphate ions, deriving from the addition of thiosulphate, released arsenate ions in the soil solution, which were thus bioavailable for plant uptake. However, it is also important to consider the interactions between sulphur and arsenic. Sulphur can promote arsenic absorption and transport to the aerial parts of the plants, since sulphur plays an anti-stress role in reducing the toxicity of arsenic [34]. Thiosulphate can thus act as a detoxifying agent by stimulating the defence system of plants while increasing the efficiency of phytoremediation due to competition between arsenate and sulphate ions for the same sites on the soil surfaces. One of the main drawbacks of assisted phytoextraction is the potential leaching of the contaminants solubilized by the additives used, however in our experimental conditions, no arsenic and mercury were found in the small amounts of leachate produced following the irrigation necessary for the plant growth. However, as a precautionary principle, we determined the Hg and As water extractable in soil after the first and the second growing cycles. We can suppose that the addition of thiosulphate mobilizes contaminants and the plants are able to uptake only a part of this amount.Following the T treatment, the soluble amount of Hg was around 2.5 mg kg-1 after the first growing cycle. Without further T addition, after the second growing cycle, the values decreased to about 0.7 mg kg-1 and 1.3 mg kg-1 for pots planted with B. juncea and L. albus, respectively. In the mesocosms treated twice with thiosulphate (T/T) the water soluble Hg amount in soil was about 1.5 mg kg-1 for all the pots. The results showed that a certain amount of mercury remained in the soil, bioavailable for further growing cycles essentially in the rhizosphere. In fact, water extractable Hg decreased after the second growth. These results are in accordance with previous findings [21] that reported an increase in soluble mercury in the rhizosphere following thiosulphate addition did not correspond to an increase in the bulk soil. The results were explained as the consequence of mercury complex decomposition with the production of sulphate ions and subsequently a decrease in mobile mercury species [21].Similar results were obtained for As. Following the T treatment, the water extractable As after the first growing cycle accounted for 1.4 and 0.91 mg kg-1 for B. juncea and L. albus mesocosms, respectively. After the second growing cycle without any further T addition (T/nt), these extractable amounts decreased slightly to 0.80 and 0.65 mg kg-1 in the pots planted with B. juncea and L. albus, respectively. The amounts increased slightly to 1.30 and 0.80 mg kg-1 when the T treatment was repeated (T/T) in the pots planted with B. juncea and L. albus, respectively. This procedure, which involves H2O extraction at the end of the growing cycle, may provide a linkage between plant uptake and the residual bioavailable fraction of the two metals. This is useful when planning other growing cycles aimed at reducing and possibly eliminating the amounts of bioavailable Hg and As which are the most dangerous for humans and the environment [25]. Unlike the use of chelating agents such as EDTA, it is not necessary to apply large amounts of thiosulphate to counteract the co-solubilization of Ca due to the low solubility of CaSO4. The risk of additive leaching is negligible, in contrast to the case of poorly biodegradable chelating agents, such as EDTA. Adverse effects on soil quality can be ruled out since the decomposition of thiosulphate produced sulphur and sulphate, which are essential for fertility.The efficiency of phytoextraction in cleaning up metal-contaminated soils has been questioned due to the long time required to reach the remediation target based on total metal concentration. However, not all the total metals in soils may be involved in environmental processes. There is growing interest in using phytoextraction to reduce only the bioavailable fractions, which are the most dangerous to the environment and human health. The clean-up time can thus be substantially shortened and the imbalance between the potential of phytoextraction and full-scale applicability can be overcome [20].The potential use of plants for phytoextraction can be derived from the ratio between the concentration of Hg and As in the shoots and the total concentration of the two elements in soil. This relationship is often called the phytoextraction coefficient (PEC) or translocation factor (TF). If the aim of phytoextraction is to remove the bioavailable fraction, this coefficient is calculated as the ratio between the concentration of the metal in the aerial parts of the plants and the bioavailable concentration in the soil. This ratio has been defined bioavailability factor (BF) [35].Table 5 summarizes the BF data considering the ratio between the concentration in the plant shoots following each treatment and the potential bioavailability of the contaminants determined by the use of 0.27 M thiosulphate. As previously reported, the As extractable by 0.27 M thiosulphate was the same as that extracted by 0.05 M phosphate.
|
4. Conclusions
- Hg and As are widespread in contaminated sites and are often present simultaneously thus entailing separate remediation strategies, due to their different chemical characteristics and different behaviour in relation to soil properties. Among clean-up procedures, phytoextraction may be a viable clean up procedure based on the sustainability and conservation of natural resources. High biomass plant species could be used together with a modification of soil properties by chemical additives, which increase the bioavailability of the contaminants (assisted phytoextraction). In this study, an innovative approach based on the use of a single mobilizing agent, ammonium thiosulphate, was tested at a greenhouse scale. Our results show that the addition of thiosulphate to the soil greatly promoted the uptake and translocation of both contaminants in the aerial parts of B. juncea and L. albus. Thiosulphate, which is specifically used to mobilize Hg in soil, was also particularly efficient in mobilizing As in this soil.The comparison between phosphate (specific mobilizing agent for As) and thiosulphate in the As extraction efficiency showed that they extracted the same amounts of this contaminant from the soil. Thiosulphate has no adverse effects on the environment since it is a common fertilizer used to promote plant growth and at the same time, it is able, with different mechanisms, to increase the bioavailability of both Hg and As. The use of the same mobilizing agent to clean up soil contaminated simultaneously with both Hg and As could be of great interest in the phytoextraction process by greatly reducing both time and costs.
ACKNOWLEDGEMENTS
- This study was supported by Eni S.p.A, Research & Technological Innovation Department, San Donato Milanese (Italy). Contract n°. 3500023724 and fully funded by Syndial S.p.A.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML