-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2015; 5(3): 90-96
doi:10.5923/j.re.20150503.02
The Use of Compost – Red Gypsum Mixture as a Low Cost Alternative Adsorbent for Lead
Gianniantonio Petruzzelli, Manuele Scatena, Irene Rosellini, Francesca Pedron
Institute of Ecosystem Study, CNR, Via Moruzzi 1, Pisa, Italy
Correspondence to: Gianniantonio Petruzzelli, Institute of Ecosystem Study, CNR, Via Moruzzi 1, Pisa, Italy.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Red gypsum, a waste material which derives from the production of titanium dioxide with a high content of CaSO4 and Fe(OH)2, was evaluated as a low-cost adsorbent mixed with green compost. Red gypsum is becoming interesting because of the widespread TiO2 industrial production in many industrialized countries. The aim of this work was to evaluate whether a mixture of red gypsum and compost could be used as an adsorbing material to decontaminate waters polluted with heavy metals. Batch adsorption experiments were carried out with Lead as representative of heavy metals in polluted waters. Lead adsorption was studied to test the performance of the composite material as a function of the composition of the mixture. The evaluation of the adsorption capacity of the compost plus red gypsum mixture was based on the Freundlich and Langmuir equations. The results indicate that the composite material has a high adsorption capacity for Pb from aqueous solutions. The adsorption capacity grew following the addition of increasing amounts of red gypsum due to the increase in negative charges, which promoted the attraction towards the positively charged Pb ions. Pb adsorption may occur as a result of different mechanisms such as ion exchange, surface complexation and electrostatic interaction. The mixture compost - red gypsum showed a considerable capacity to remove Pb from aqueous solutions over a wide range of concentrations.
Keywords: Adsorption, Red gypsum, Compost, Lead, Freundlich, Langmuir
Cite this paper: Gianniantonio Petruzzelli, Manuele Scatena, Irene Rosellini, Francesca Pedron, The Use of Compost – Red Gypsum Mixture as a Low Cost Alternative Adsorbent for Lead, Resources and Environment, Vol. 5 No. 3, 2015, pp. 90-96. doi: 10.5923/j.re.20150503.02.
1. Introduction
- Water contamination by heavy metals arising from industrial activities is a problem for all industrialized countries. Galvanic industries, mining activities and textile productions are only some examples of the many industrial processes that can release heavy metals in wastewater. Worldwide industrialization seriously contributes to the release of heavy metal polluted streams. Unlike organic compounds, heavy metals are not biodegradable and can accumulate in living organisms, causing various diseases and disorders. They must thus be removed before they can be discharged into water bodies. Very strict regulations are enforced worldwide to prevent the discharge of toxic heavy metals into aquatic systems. The removal of heavy metals from wastewaters is thus an environmental issue of paramount importance [1]. Adsorption is one of the best techniques to clean-up heavy metals from aqueous solutions such as waste water, stormwaters, landfill leachates and acid mine drainage [2]. This technology is particularly appreciated for its consolidated affordability and public acceptance. One of the most important aspects of the adsorption processes is the possibility to use low cost materials as adsorbents and simultaneously promote waste recycling [3]. Among these possibilities, compost is particularly interesting because it can act as “bio-adsorbent” resulting from the aerobic biological degradation of organic waste [4, 5]. The utilization of compost in agriculture is a diffuse practice to improve the physical properties and fertility of the soil and at the same time is a practical sustainable and efficient option in the general problem of waste management. Other waste materials that can act as adsorbents are also being studied and their use in decontaminating polluted waters can play a very important role in solving the problem of their final destination [6, 7]. Red gypsum is a waste material that derives from the production of titanium dioxide, a widespread industrial activity in many industrialized countries. The most important producers of pigments based on TiO2 are located in Asia and North America. Asia-Pacific is also the principal sales market for titanium dioxide with 40% of total global demand. The production of titanium dioxide by the sulphate method in which ilmenite (FeTiO3) is treated with sulfuric acid 98% for its dissolution, resulted in the production of an acidic effluent [8]. Red gypsum is mainly constituted by CaSO4 2H2O, and has also a high concentration of iron hydroxides according to the simplified following scheme:FeTiO3+2H2SO4→ TiOSO4+2H2O + FeSO4TiOSO4+H2O → TiO2n·H2O + H2SO4This acid solution, after TiO2 precipitation, is neutralized with lime or limestone, generating large amounts of a waste product: red gypsum, so called because of the red color imparted by the iron hydroxides. This by-product is then separated by filtration.Ca(OH)2 +H2SO4→ CaSO4·2H2OFeSO4 +Ca(OH)2→ Fe(OH)2 +CaSO4The final disposal of large amounts of red gypsum generates costs that impact on industrial competitiveness. Thus, the evaluation of the adsorption properties of this material is a viable option of valorization of this kind of waste that should be considered. The aim of this work was to evaluate whether a mixture of red gypsum and compost could be used as an adsorbing material to decontaminate waters polluted with heavy metals. The materials were used after had been mixed thoroughly in batch experiments. Lead has been selected as representative of heavy metals in polluted waters, because it is almost totally absent in the materials used. High concentration of lead in waters deriving from several industrial activities is a major health and environmental concern since lead is dangerous even at low concentration and tends to bio-accumulate in the food chain [9].
2. Materials and Methods
- A Compost and red gypsum (RG) were derived from the Archives of Italian Institute of Soil Chemistry. The main characteristics of compost and red gypsum were determined in accordance with Chemical Methods of the Soil Science Society of America [10].Batch sorption experiments were carried out at constant values of pH (7.4) and solid/liquid ratio (1:10). A metal solution (20 mL) at different concentrations from 1 to 50 mg/L was added to 2 g of compost - red gypsum mixtures. Compost includes putrescible garden organics, woody garden organics and trees residues. The compost and red gypsum were dried at 45°C for 48 h and then ground to pass a 2 mm sieve. The mixtures were prepared by mixing compost with increasing amounts of red gypsum to obtain final materials with 10, 20 and 30% of red gypsum. The Pb solution was prepared from a NIST standard solution of 1000 mg Pb per liter in MilliQ water. Suspensions were shaken for 12 h at 20±0.5°C. Preliminary sorption experiments, showed no significant variation in Pb concentration for periods longer than 4 h. This time was considered adequate to obtain a lead distribution near the equilibrium. After equilibration, the suspensions were centrifuged at 7000 xg to separate the solution phase from the suspensions and Pb concentrations in the supernatants were determined. The amount of Pb adsorbed per mass of the different adsorbing materials was calculated by the difference between the quantity added and that recovered in the equilibrium solution, according to the equation:
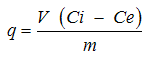 where,q = The amount of Pb adsorbed per unit mass (m) of the adsorbent expressed as mg g-1Ci = The initial Pb concentration (mg L-1)Ce = The Pb concentration at equilibrium (mg L-1)V = The volume of Pb solution added (L)All experiments were conducted in triplicate, and the mean values are reported in the text. The maximum standard deviation was ±2.3%.Pb concentration was determined using ICP-OES (Varian AX Liberty) with a method for the generation of hydrides [10]. Quality assurance and quality control were performed by testing a standard solution every 10 samples. A certified reference material (SQC001) was used to control the quality of the analytical system. The detection limit for Pb was 0.005 mg L-1. The recovery of spiked samples ranged from 95 to 101% with an RSD of 1.93 of the mean.
where,q = The amount of Pb adsorbed per unit mass (m) of the adsorbent expressed as mg g-1Ci = The initial Pb concentration (mg L-1)Ce = The Pb concentration at equilibrium (mg L-1)V = The volume of Pb solution added (L)All experiments were conducted in triplicate, and the mean values are reported in the text. The maximum standard deviation was ±2.3%.Pb concentration was determined using ICP-OES (Varian AX Liberty) with a method for the generation of hydrides [10]. Quality assurance and quality control were performed by testing a standard solution every 10 samples. A certified reference material (SQC001) was used to control the quality of the analytical system. The detection limit for Pb was 0.005 mg L-1. The recovery of spiked samples ranged from 95 to 101% with an RSD of 1.93 of the mean.3. Results and Discussion
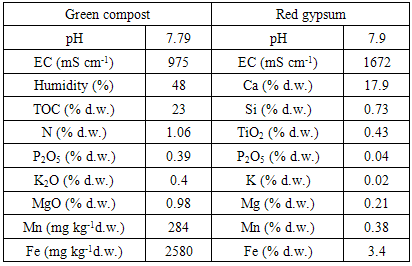
- The main characteristics of compost and red gypsum are reported in Table 1. Both the materials contained negligible Pb amounts.
|
 q = K Ce1/nwhere q is the amount adsorbed per unit mass of the adsorbent (mg g-1)Ce the equilibrium concentration of the adsorbate (mg L-1)K and n are the Freundlich constants related to adsorption capacity and intensity, respectively.
q = K Ce1/nwhere q is the amount adsorbed per unit mass of the adsorbent (mg g-1)Ce the equilibrium concentration of the adsorbate (mg L-1)K and n are the Freundlich constants related to adsorption capacity and intensity, respectively. 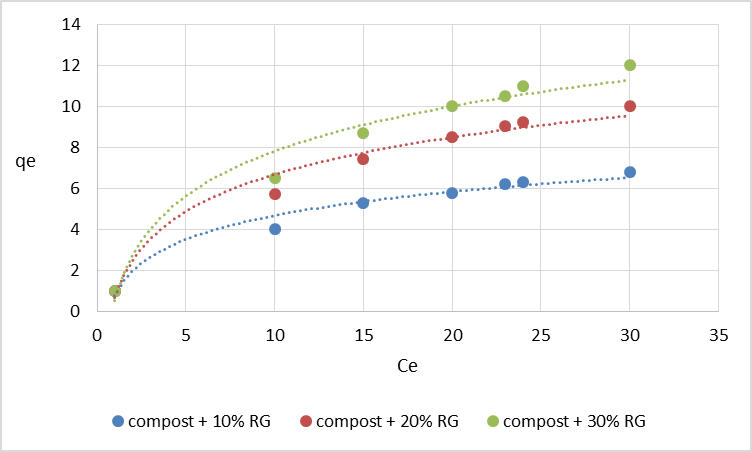 | Figure 1. Pb adsorption isotherms on the mixtures compost + red gypsum |
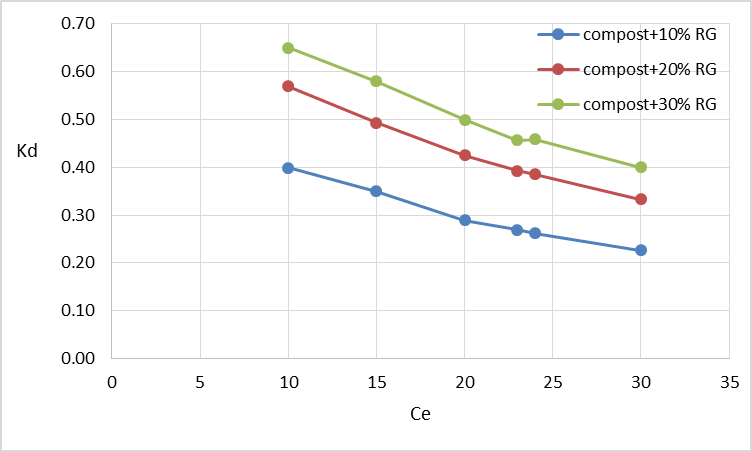 | Figure 2. Effect of increasing percentage of red gypsum in the composite material on the distribution coefficient (Kd) |
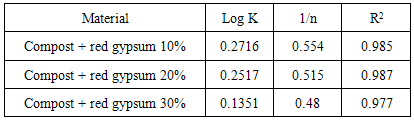
 log q = logK + 1/n log CeThe Freundlich constants, K and n, were estimated by linear regression analysis from the experimental adsorption data obtained for Pb. If 1/n>1, the adsorption can be considered as chemisorption, while for 1/n<1 the adsorption is a favorable physical process [12, 13, 14].The mean values of the Freundlich parameter are shown in Table 2.
log q = logK + 1/n log CeThe Freundlich constants, K and n, were estimated by linear regression analysis from the experimental adsorption data obtained for Pb. If 1/n>1, the adsorption can be considered as chemisorption, while for 1/n<1 the adsorption is a favorable physical process [12, 13, 14].The mean values of the Freundlich parameter are shown in Table 2.
|
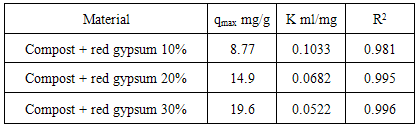
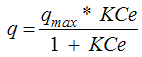 where q (mg g-1) = The amount of Pb adsorbed per unit of weight of the adsorbentCe (mg mL-1) = The equilibrium Pb concentration qmax and K = Adjustable parameters linked to the maximum and to the energy of adsorption. The Langmuir isotherm model is based on some assumptions, which limit its applicability to simple systems. In fact, the adsorbent must be characterized by a finite number of active sites per unit area, and all the sites should have the same adsorption energy and bind a single adsorbate molecule, to form a monomolecular layer of substance. Despite these constrains, the Langmuir equation is able to describe suitably all those adsorption processes that tend to reach saturation and it has been used for many years to describe soil adsorption processes, where these conditions are not met. The Langmuir equation has also been used to describe heavy metal adsorption on unconventional adsorbents, such as red mud, fly ash, etc., without assigning an exact chemical configuration to the complex adsorbing surfaces [6]. Thus, although this model has some theoretical limitations, since the bonding energy at the sorption sites cannot be considered uniform, it enables the adsorption maxima to be compared for different materials. The Langmuir equation provides a quantitative evaluation of the effects of increasing the amounts of red gypsum on the sorption capability of this composite material. Data from the Langmuir equation are reported in Table 3.
where q (mg g-1) = The amount of Pb adsorbed per unit of weight of the adsorbentCe (mg mL-1) = The equilibrium Pb concentration qmax and K = Adjustable parameters linked to the maximum and to the energy of adsorption. The Langmuir isotherm model is based on some assumptions, which limit its applicability to simple systems. In fact, the adsorbent must be characterized by a finite number of active sites per unit area, and all the sites should have the same adsorption energy and bind a single adsorbate molecule, to form a monomolecular layer of substance. Despite these constrains, the Langmuir equation is able to describe suitably all those adsorption processes that tend to reach saturation and it has been used for many years to describe soil adsorption processes, where these conditions are not met. The Langmuir equation has also been used to describe heavy metal adsorption on unconventional adsorbents, such as red mud, fly ash, etc., without assigning an exact chemical configuration to the complex adsorbing surfaces [6]. Thus, although this model has some theoretical limitations, since the bonding energy at the sorption sites cannot be considered uniform, it enables the adsorption maxima to be compared for different materials. The Langmuir equation provides a quantitative evaluation of the effects of increasing the amounts of red gypsum on the sorption capability of this composite material. Data from the Langmuir equation are reported in Table 3.
|
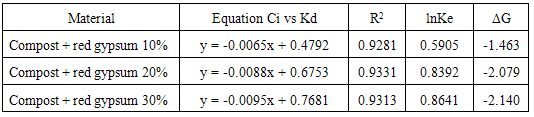
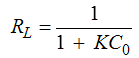 Where K is the Langmuir constant and C0 is the highest initial Pb concentration (mg L-1). The value of RL indicates the type of adsorption either to be favorable (0 < RL <1) or unfavourable (RL >1) [20, 21, 22, 23]. Values of the dimensionless constant RL were found to be within the favorable limits [24] for all the isotherms as they ranged from 0.16 (compost + R.G. 10%) to 0.79 (compost + R.G. 30%). The data showed that the affinity of the adsorbents for Pb ions increased with increasing the amount of RG added.The higher adsorption capacity of the materials following the addition of increasing RG amounts could be explained by an increase in the surface sites available for adsorption. This effect can be highlighted by using the distribution coefficient Kd defined as the ratio of Pb concentration in the solid phase and in solution at equilibrium (Figure 2).Increasing the amount of added red gypsum the distribution coefficient Kd of lead tended to increase at all the equilibrium concentrations. The constituents of red gypsum increased negative charge on the surface and it promoted the attraction towards the positively charged Pb ions. The result indicates that all the composite materials are characterized by a high adsorption capacity for Pb from aqueous solution.By plotting Kd values versus the initial concentration Ci, it is possible to obtain the equilibrium constant Ke as the intercept of the obtained straight lines reported in Table 4 together with the corresponding R2. The free energy of adsorption reactions (△G) on the composite materials can be calculated according to the equation:
Where K is the Langmuir constant and C0 is the highest initial Pb concentration (mg L-1). The value of RL indicates the type of adsorption either to be favorable (0 < RL <1) or unfavourable (RL >1) [20, 21, 22, 23]. Values of the dimensionless constant RL were found to be within the favorable limits [24] for all the isotherms as they ranged from 0.16 (compost + R.G. 10%) to 0.79 (compost + R.G. 30%). The data showed that the affinity of the adsorbents for Pb ions increased with increasing the amount of RG added.The higher adsorption capacity of the materials following the addition of increasing RG amounts could be explained by an increase in the surface sites available for adsorption. This effect can be highlighted by using the distribution coefficient Kd defined as the ratio of Pb concentration in the solid phase and in solution at equilibrium (Figure 2).Increasing the amount of added red gypsum the distribution coefficient Kd of lead tended to increase at all the equilibrium concentrations. The constituents of red gypsum increased negative charge on the surface and it promoted the attraction towards the positively charged Pb ions. The result indicates that all the composite materials are characterized by a high adsorption capacity for Pb from aqueous solution.By plotting Kd values versus the initial concentration Ci, it is possible to obtain the equilibrium constant Ke as the intercept of the obtained straight lines reported in Table 4 together with the corresponding R2. The free energy of adsorption reactions (△G) on the composite materials can be calculated according to the equation: △G = -RTlnKeWhere R = gas constant (0.008314 kJ mol-1 K-1) and T = temperature in Kelvin (K)Also these data are reported in Table 4.
△G = -RTlnKeWhere R = gas constant (0.008314 kJ mol-1 K-1) and T = temperature in Kelvin (K)Also these data are reported in Table 4.
|
4. Conclusions
- Studies regarding the use of unconventional adsorbents for metal retention are of increasing interest in the latest years. Using available, low-cost, efficient adsorbents is one of the most important aspects of successful adsorption processes. Compost has been successfully used for adsorbing organic and inorganic contaminants [28, 29, 30] and gypsum is considered as an affordable adsorbent for contaminated waste water [31, 32]. The compost plus red gypsum mixture showed a considerable ability for the removal of Pb from aqueous solutions over a wide range of concentrations. The results obtained showed that the composite materials are characterized by an interesting overall adsorption capacity as revealed by the values of adsorption maxima, which compare well with adsorption capacity of other low cost sorbents for heavy metals. The simultaneous presence of compost and red gypsum is likely to produce an articulated porosity in the composite material. The mesopores could facilitate the transport of lead to the smallest pores, and the micropores produce a significant increase in the specific surface area and therefore of the adsorbing capacity. Due to the heterogeneous nature of this composite material, the Pb adsorption may occur as a result of different mechanisms such as ion exchange, surface complexation, and electrostatic interaction. Not all the kinds of reactions involved in the adsorption can be identified from adsorption data. As previously stated [33] complete understanding of the sorption mechanism can only be achieved by using spectroscopy techniques such as extended X-ray absorption fine structure EXAFS, or resonance anomalous X-ray. For example, the characteristics of the composite material also suggest the possibility of the formation of ternary metal-bridged complexes in which Pb is bonded to both inorganic and organic reactive surfaces, thus increasing the total Pb adsorption [6]. The composite material used in this work was obtained by the mechanical mixing of compost and red gypsum, further researches should be carried out to optimize the preparation of this bio-inorganic material in order to increase its capacity to remove heavy metal and to deepen the knowledge of mechanisms involved.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML