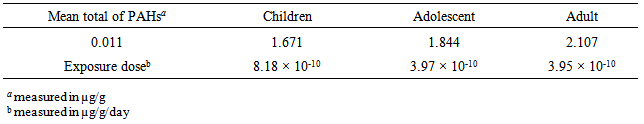-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2014; 4(6): 247-259
doi:10.5923/j.re.20140406.01
Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Media: Factorial Effects and Human Dietary Exposure Risk Assessment
M. O. Obiakor1, J. C. Okonkwo2, C. D. Ezeonyejiaku3, C. N. Okonkwo1
1School of Environmental and Rural Science, University of New England, Armidale, NSW, Australia
2Department of Animal Science and Technology, Nnamdi Azikiwe University, Awka, Anambra, Nigeria
3Department of Zoology, Nnamdi Azikiwe University, Awka, Anambra, Nigeria
Correspondence to: M. O. Obiakor, School of Environmental and Rural Science, University of New England, Armidale, NSW, Australia.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Polycyclic aromatic hydrocarbons (PAHs) present in water column and preponderant fish species of Anambra River were conducted. Eight PAHs (naphthalene, acenaphthene, fluorine, phenanthrene, anthracene, pyrene, fluoranthene and benzo [a] pyrene) classified as priority pollutants by the United States Environmental Protection Agency and World Health Organisation were measured in the media following solid-phase extraction and gas chromatographic procedures on three factorial experimental model designs. Season was tested at two levels: rainy and dry; fish species at two levels: Synodontisclarias and Tilapia nilotica; and location factor at five levels: Enugu Otu, Ezi-Aguleri, Otuocha, Otu-Nsugbe, and Onono. The human PAH exposure risk was assessed and daily intake through fish consumption determined. Polycyclic aromatic hydrocarbons were found preponderant in the water and fish species for both rainy and dry seasons at the five distinct locations. Concentrations detected in these principal media were generally low compared to earlier studies and unaffected by the season, location and fish species, including all the factorial level interaction effects. However, the dietary intakes of the contaminant through fish consumption were a subject of public health concern on continuous daily exposure as the PAH concentrations in the examined fish sourced from some of the locations in different seasons were higher than stipulated values by Environment Canada for edible tissues of fish and/or shellfish for human consumptions. Effective and continued monitoring of the quality conditions of the river needs to be sustained and compared with baseline catchment data for quality deviation as crude oil production is expected to commence at this delicate site. Further quantifications of these organic substances in other fauna and investigation in entire flora of the river, and modelling of its trophodynamics would practically underpin sound management strategies and sustainability of the aquatic resources.
Keywords: Anambra River, PAHs, Water quality, Fish species, Aquatic pollution, Dietary exposure, Public health
Cite this paper: M. O. Obiakor, J. C. Okonkwo, C. D. Ezeonyejiaku, C. N. Okonkwo, Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Media: Factorial Effects and Human Dietary Exposure Risk Assessment, Resources and Environment, Vol. 4 No. 6, 2014, pp. 247-259. doi: 10.5923/j.re.20140406.01.
Article Outline
1. Introduction
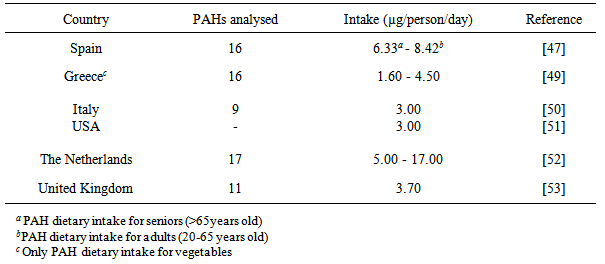
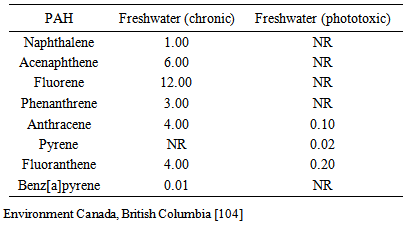
- Polycyclic aromatic hydrocarbons (PAHs) are organic chemical compounds that can occur naturally in the environment but their occurrence can be accelerated by anthropogenic activities. They are important emerging persistent environmental contaminants due to their existence in various combinations and ubiquity. Polycyclic aromatic hydrocarbons as priority organic pollutants (POPs) have in recent times steadily increased [1] and are widespread in the environment [2, 3], occurring in food, air, water, soil, and sediments [4-8].The solubility of PAHs decreases in water with increasing molecular weight [9-11] resulting to its low concentration in water column [12, 13]. Because of its hydrophobicity, the presence of PAHs in surface water or groundwater indicates pollution [11]. Certain PAHs occur in the environment at low concentrations due to their low biodegradability, and persist with elimination difficulties [14] while the halogenated forms tend to be chemically stable [15]. PAHs are rapidly adsorbed onto particles especially the heavier compounds in the surface water with the hydrophobic elements readily attaching to the bottom sediments [14, 16]. They become much more stable than the pure compounds on adsorption and resistant to sensitive photo-chemically induced processes of oxidation and nitration reactions [17].Natural and anthropogenic activities and emissions remain the sources of PAHs in both terrestrial and aquatic environment. Forest fires, volcanoes, and microorganisms (e.g., bacteria and fungi) demonstrate the natural sources while combustion of fossil fuels, crude oil and petroleum spills, industrial and municipal waste and wastewaters represent the anthropogenic environmental inputs [1, 18-22]. The PAH types present in water give information on the derivative sources of the organic contaminants [23-25]. Reference to the documentary report of Simpson et al. [23], PAHs of biogenic origin such as perylene and retene are almost produced entirely from organic matter and are good markers of terrestrial organic inputs. However, existence of larger levels of lower molecular weight PAHs such as fluorene and acenaphthene in environmental media implies natural or petrogenic PAH contamination; while prominent concentrations of higher molecular weight and membered-ring PAHs (e.g., fluoranthene, phenanthrene, pyrene) and fewer low molecular weight PAHs demonstrate combustion or pyrolytic origins [26].The liphophilic nature of PAHs makes it easier to penetrate biological membranes and accumulate in organisms [15]. Because of their public health effects, United States Environmental Protection Agency (U.S EPA) and World Health Organisation (WHO) classified sixteen PAHs as priority pollutants and considerable number (e.g., benzo[a]pyrene, chrysene) as potential human carcinogens. Its mutagenicity and carcinogenicity have been experimentally reported [27-29]. Various bio-effects of PAHs have been properly demonstrated and documented on a large scale such as interactive effects on DNA and RNA [30-34]; haematological parameters [35, 36]; hepatic lesions and changes [37-39]; reproductive abnormalities [40, 41]; immune-suppression [36, 42, 43]; and developmental toxicity [44]. PAH biomarker systematics and biocycle in fish have been extensively reviewed by Tuvikene [15].Baseline determination of PAH occurrences and concentrations in the aquatic environment provides information on the original state of the media and scale of organic pollution. Consequently, many common PAHs have short residence time in water column due to volatilization and oxidation, and can quickly be eliminated from the system [45]. The presence in the water and other media therefore denotes current or chronic pollution [46] and provide overview of possible human exposure to the contaminant and consequent toxicity.Dietary intake of environmental contaminants is a public health concern as most of the food are very essential for body growth such fish and other aquatic species. Seventy percent of exposure to PAHs arises from the diet [47]. Doll [48] commentary showed that considerable number of epidemiological studies demonstrated substantial proportion of human cancers to be linked, in part, to dietary exposure. Studies have shown varying concentrations of PAHs in diets and detailed the likelihood of human exposure and toxicity as most of these organic chemicals are potential carcinogens (Table 1).
|
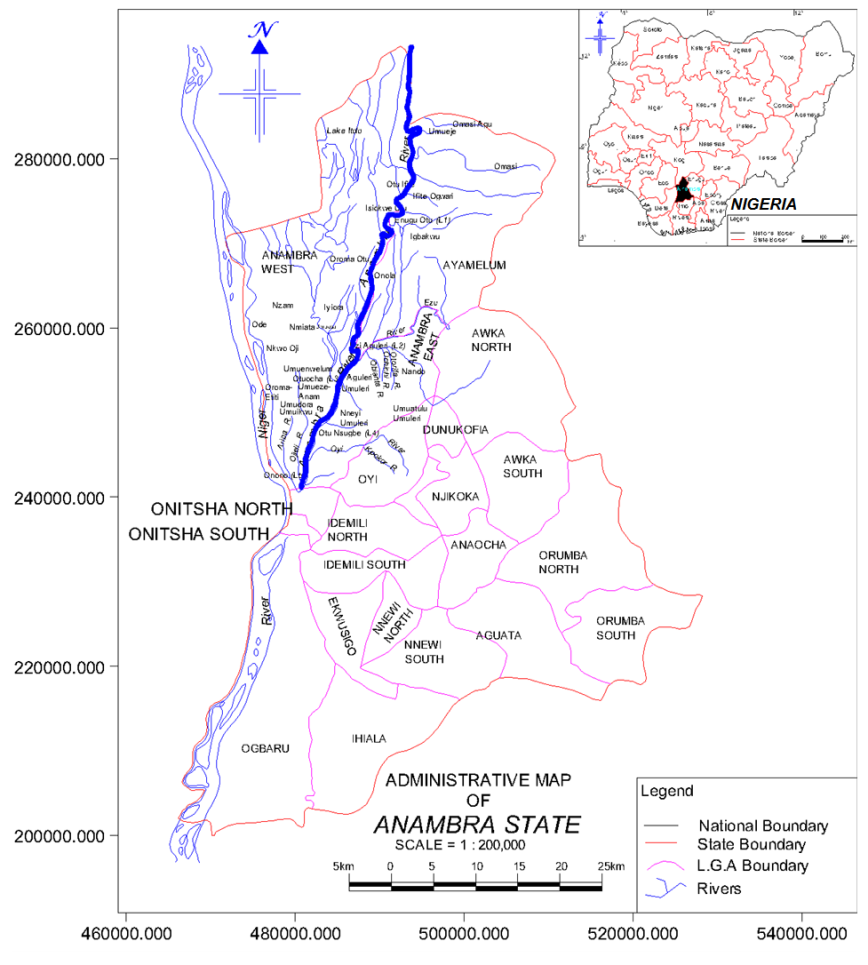 | Figure 1. Map showing Anambra River and sampling locations |
2. Materials and Methods
2.1. Study Area
- The study area is Anambra River, located in Anambra State of Nigeria. Anambra State lies between latitude 5° 40’N and 6° 45’N and longitude 6° 35’E and 7° 21’E. The climate is tropical with average annual rainfall of 2000 mm and mean temperature of 27℃ [55]. Anambra River spatially lies between latitudes 60 00’N and 60 30’N and longitudes 6° 45’E and 7° 15’E. The river is at the South Central region of Nigeria, close to the east of the Niger River into which it empties [67]. Anambra River is approximately 207.4 km to 210 km in length [68, 69], rising from the Ankpa hills (ca. 305-610m above sea level) and discharging into River Niger at Onitsha [68]. The entire River basin drains an area of 14014km2 [67], (Figure 1).
2.1.1. Geology and Climate
- The River is geologically underlain by cretaceous sedimentary rock [67]. The basin is situated at the southern extremity of the Benue Trough of Nigeria, bounded on the west by the Precambrian Basement Complex Rocks of Western Nigeria and on the East by the Abakaliki Anticlinorium [70]. Anambra River rises from the Ankpa Hills and discharges into River Niger [67, 68, 71]. The river receives many river tributaries that form its river basin, forming a dendritic drainage pattern.There are two main seasons, the dry season (October / November-March) and the rainy season (April-September / October) approximately corresponding to the dry and flood-phases, respectively of the hydrological regime [71]. It is affected by the movement of the inter-tropical convergence zone (ITCZ), the boundary zone between the dry continental air mass of the Sahara and the moist maritime air mass from the Atlantic Ocean. Seasonal shifts in the position of this boundary zone are responsible for the cycle of rainy and dry season weather observed in this area. Generally temperature is highest and rainfall lowest from January to March. In addition, temperatures tend to be higher and rainfall and humidity lower as one moves North in the river basin. This is due to a combination of increasing distance from the maritime air mass over the Atlantic Ocean and increasing elevation [67]. The water temperature and Secchi disc reading in the river ranges from 24℃ to 31℃ and 5cm to 85cm, respectively [68]. The mean annual rainfall of the river is between 150cm and 200cm [72].
2.1.2. Land Use and Land Cover
- Anambra Basin is one of the richest, if not the richest area for agricultural and fishery production in the Nigerian Lower Niger [73,74]. Principal crop products include a wide variety of large yams (Dioscoreaspp), sweet potatoes, cassava, and rice; while clariids, gymnarchus, and mormyrids dominate fish production, which are available throughout the year [67]. The river has fifty-two species belonging to seventeen families; 171, 236 and 169 individuals at Ogurugu, Otuocha and Nsugbe stations, respectively. Two families, Characidae, 11.5% and Mochokidae, 11.8%, constitute the dominant fish families in the river. The dominant fish species were Synodontisclarias 6.9%, Macrolepidotus curvier 5.7%, Labeocoube 5.4%, Distichodusrostrtus 4.9% and Schilbemystus 4.5% [71]. Members of Ardeidae aquatic animal family were the most abundant and they were followed by Acciptridae while Sirenia were the least occurring in the river. The most abundant animals utilizing the river were the Ardeacinora, with 22.2% occurrence and this was followed by Caprini spp. With 13.5% and Varanusniloticus with 10.04%. The least abundant animals utilizing the river were Chephalophusrufilatus and Erythrocebuspetas with 0.58% of occurrence each [71].Anambra River serves as the main source of potable water in the areas surrounding it, as it is common for residents to fetch drinking water there. There is currently no treatment of this source of drinking water, either done by government agencies or by individuals in their private homes before usage [58].
2.2. Research Design
- The first approach of the study was designed to assess the PAHs concentrations in fresh water column and preponderant fishes in the River. Concentrations of PAHs in water column were examined under 2x5 experimental design, while the concentration of PAHs in fishes was conducted under 2x2x5 factorial design to test the effects of season, species (species) and location on the Polycyclic Aromatic Hydrocarbons (PAHs) concentrations in Freshwater column and fishes. Season was tested at two levels, viz: rainy season and dry season; species was handled in two levels namely Synodontisclarias, Linnaeus, 1758 and Tilapia nilotica, Linnaeus, 1757 while five locations namely Enugu Otu, EziAguleri, Otuocha, Out Nsugbe and Onono were studied.The model used is:Yijkl = μ+SSi+Lj+Sk+SSLij+SSSik+LSjk+SSLSijk+ЄijklWhere;Yijkl = PAHs (naphthalene, acenaphthene, fluorine, phenanthrene, anthracene, pyrene, fluoranthene and benz [a] pyrene) values that were observed due to:μ = the population mean;SSi = the effect of ith seasonLj = the effect of jth location from where the samples were collectedSk = the kth species effect;SSLij, SSSik, and LSjk = are the interactions between season and location, season and species, and species and location, respectively.SSLSijk = the third level interaction between season, location and species.Єijkl: is the error term associated with the experimentation.Assumptions: error term is independently, identically and normally distributed with zero mean and constant variance. That is, iind (0, σ2).Grid sampling technique as described by Gelderman et al. [75] was modified to fit aquatic environment and used for sample collection. Each location or station was divided into rectangular grids and samples were collected from various grids. Total of twenty-four fishes, comprising twelve Synodontisclarias (Linnaeus, 1758) and twelve Tilapia nilotica (Linnaeus, 1757) were used for each location.
2.3. SamplingSites
- The experimental site comprised of five distinct locations/stations established to cover possible impacted and unimpacted area of both commercial and urban activities along the river course based on an earlier field reconnaissance tour. The locations (LX) of the various sampled stations are (Figure 1):L1 = Enugu Otu: rice production and farming siteL2 = EziAguleri: farming, fishing, and effluent discharge;L3 = Otuocha: rice production, marketing activities, and solid waste disposal;L4 = OtuNsugbe: wastewater effluent discharge, farming, and marketing activities; andL5 = Onono: agricultural activities, fishing and sewage disposal. The location is close to Onitsha metropolis and mouth of Oyi River (Figure 1), the repository of industrial effluents and municipal/ domestic wastes.
2.4. Sample Collection and Preparations
- In line with the experimental design, samples were collected in mid rainy and dry seasons (mention months of mid rainy and dry seasons and year here). For each season, samples were collected at five distinct locationsnamely; 1, 2, 3, 4 and 5. In situ measurements of some physicochemical parameters were carried using appropriate digital read-out meters before the samples were taken to laboratory for detailed analysis. The parameters measured in situ included temperature, pH, electrical conductivity, total dissolved solid, dissolved oxygen, and turbidity.
2.4.1. Collection of Fish and Water Samples
- Live Synodontisclarias (Linnaeus 1758) and Tilapia nilotica (Linnaeus 1757) of fairly similar live-weight irrespective of sex and age were collected from Anambra River at the five stations using set nets, long-lines and traps. The fish were stored at temperature of -20℃. Two litres of water were collected at each station using grid technique with wide mouth polyethylene containers thoroughly cleaned. Surface water was collected at the depth of 0.5m. Total of six samples constituted routine collections at designated grids (points) to determine fluctuations of the parameters measured in situ and twenty samples for PAH evaluations. Samples of water were preserved by adding drops of nitric acid at pH < 2 [76] and stored below 4℃ in a refrigerator prior to analysis. The relative distance between each station is approximately 12km (Figure 1). All the sample collections were made during the morning hours in both seasons.
2.4.2. Sample Preparations
- The water was analysed separately before the fish samples following the methods described by Kanchanamayoon and Tatrahun [14]. Prior to extraction, the fish specimens were dissected and the muscle tissue removed. 10g of muscle was grounded with anhydrous sodium sulphate until completely dry homogenate was obtained. Extraction was carried out with dichloromethane in a cold extraction mode. After the extraction, the extracting solvent is evaporated using rotary evaporator and the mass of the extractable fat determined by gravimetry.
2.5. Determination of PAHs
2.5.1. Sample Clean up
- The isolation of PAHs from the lipid matrix was done by solid phase extraction in normal phase mode. Activated silica gel was loaded into a glass chromatographic column (id 20mm, height 400mm) and conditioned with dichloromethane. The extractable fats from the samples were dissolved in 5ml n-hexane and loaded in column and eluted with 60ml n-hexane. The eluents were then concentrated using a rotary evaporator and under a gentle stream of pure nitrogen. The samples were thereafter dissolved in 1ml acetone and ready for gas chromatographic (GC) analysis.
2.5.2. Gas Chromatography
- Analysis was performed with Perkin Model 5890 gas chromatograph equipped with Ni 63 electron capture detector. Nitrogen was used as a carrier gas at a flow rate of 40ml/s. Data obtained were processed using Hewlett Packard 3396 integrator. The operating parameters were as follows; injector temperature set at 250℃ and 300℃ for the detection, the oven temperature programmed at 150℃ initially (5 min hold) and then increased to 300℃ at 4℃/min to give the analysis a period of 34min.Retention time match with those of the standards identified PAHs congeners in the water and fish. The limit of detection for individual PAHs was 0.001 for both water and fish and recovery yields were upto 90%.
2.6. Human PAH Exposure Risk Assessment
- During the experimental period, subsets of the human population comprising of children, adolescents and adults living at the Anambra River were randomly sampled to determine their fish consumption rate (Table 6). The fish employed in our studies are widely consumed by the local residents and intake quantifications were based on the species. Because of the low literacy level in the area, interview method was used whereby the individuals were descriptively interviewed on the quantity of fish consumed per day and their body weights consensually taken by flexible weight equipment. Total of two hundred and seventy three individuals were interviewed irrespective of gender due to in part, the customary restrictions on access to some people and other factors relating to safety of the enumerators. The daily intake of PAHs from fish consumption was calculated following the formula [47];
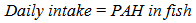
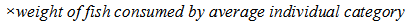 Exposure doses from ingestion of fish among the population were derived following the equation of Agency for Toxic Substances and Disease Registry (ATSDR) on calculation of exposure doses assuming that all fish consumed are caught from one contaminated water body:
Exposure doses from ingestion of fish among the population were derived following the equation of Agency for Toxic Substances and Disease Registry (ATSDR) on calculation of exposure doses assuming that all fish consumed are caught from one contaminated water body: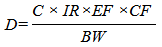 Where,D = Exposure dose (µg/g/day)C = Contaminant concentration (µg/g)IR = Intake rate of contaminated medium (µg/day)EF = Exposure factor (unitless) -the fish intake rate is a daily average, so the exposure factor is equal to 1CF = Conversion factor (10-6 µg/g)BW = Body weight
Where,D = Exposure dose (µg/g/day)C = Contaminant concentration (µg/g)IR = Intake rate of contaminated medium (µg/day)EF = Exposure factor (unitless) -the fish intake rate is a daily average, so the exposure factor is equal to 1CF = Conversion factor (10-6 µg/g)BW = Body weight2.7. Data Analysis
- Data obtained were analysed using SPSS (version 17) and Microsoft Excel (2007 version). Significance and interaction factorial effects on PAH concentrations detected in the principal media sampled were subjected analysis of variance (ANOVA) and the comparisons among treatment means were conducted using Post Hoc LSD since equal variances were tenable. The limit of significance was P<0.05.
3. Results
3.1. Physicochemical Characteristics of Water Column
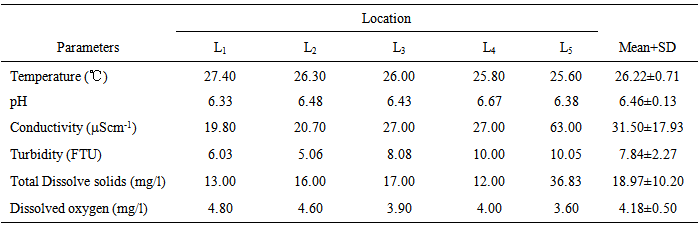
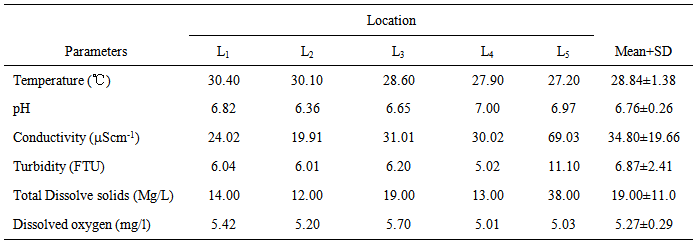
- The results of the in situ measurements of the physicochemical characteristics of water column from the Anambra River in mid-rainy and –dry seasons prior to PAHs quantification are shown in Tables 2 and 3.
|
|
3.2. Polycyclic Aromatic Hydrocarbon (PAH) Concentrations in Anambra River
- Shown in Tables 3 and 4 are the concentrations of eight PAHs detected in the two principal media of the Anambra River. Generally, the concentrations of PAHs detected in the sampled media of the river were low in both seasons for fish species and water column. Water samples contained smaller concentrations of PAHs, and varied among sampling seasons and locations. The effects of season and location on PAH concentrations in water column are presented in table 3 while season, species, and location effects for the fish are given in table 4. There was no significant (P>0.05) effect on the concentrations of the PAHs resulting from the season and location; and season, species/species and location factors for water column and fish, respectively. Acenaphthene and fluorathene were not detected in the water column throughout the sampling regime. Phenanthrene concentration was only observed in dry season at Onono (L5) (Table 4).
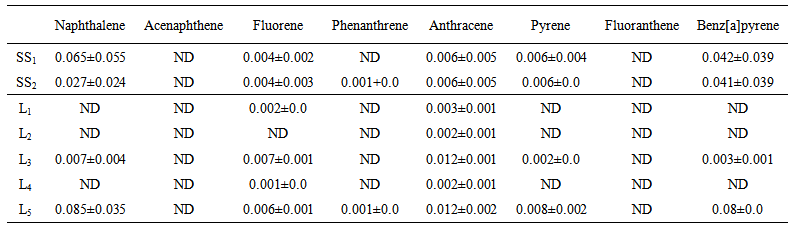 | Table 4. Season and location effects on the PAH (ng/l) concentrations in water column |
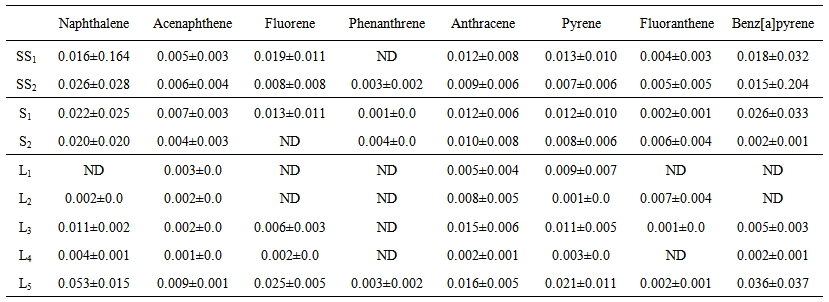 | Table 5. Seasons, species and location effects on the mean (±S.E) PAH concentrations (µg/g) in fish |
3.3. Polycyclic Aromatic Hydrocarbon Human Exposure Risk Assessment
- Table 6 summarizes the demographic parameters and fish consumption rate of the sampled population residing at the Anambra River. The daily intakes of PAHs through fish consumption and exposure doses among children, adolescent and adult are presented in Table 7.
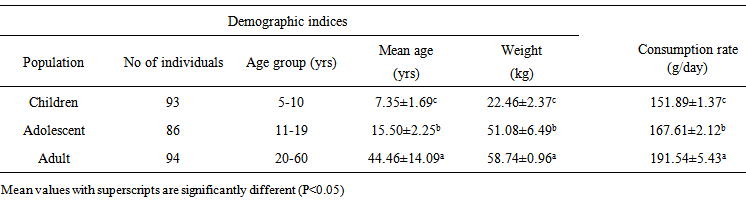 | Table 6. Enumerated fish consumption rate among the sampled population of the Anambra River |
|
4. Discussion
- The PAH concentrations in the Anambra River provide comprehensive look at present water quality conditions. One of the aims of this work was to empirically investigate the PAH levels in different environmental media of the river. Polycyclic aromatic hydrocarbons were detected in various locations of the river but existed at very low concentrations in both seasons with numerical differentiations compared to the records of earlier studies and water quality criteria given by Environment Canada (on Benzo[a]pyrene) for the sampled water column (see appendix). Due to low solubilities and high octanol-water partition coefficients, PAHs often have short residence time in water. Polycyclic aromatic hydrocarbons of lower molecular weight (1-3 ringed) are usually lost due to microbial degradation and volatilisation while larger molecular weight compounds (4-5 ringed) get lost as a result of photo-oxidation and maybe attached to the underlying sediments [46, 77].Comparatively low detection of the contaminant level in fish species to previous investigations may stem from their inherent homeostatic mechanisms of metabolising xenobiotics. Fish has a physiological mechanism of rapid PAH biotransformation [78, 79], which are influenced by various factors such as chemical exposure route and time, lipid content of tissues, environmental factors, exposure to multiple contaminants, and differences in species, age, sex [80], and health conditions of the test animals. The biotransformation of hydrophobic-containing substances in fish is a major determinant of its toxicity, distribution and excretability [15]. The concentrations in fish were higher than water column because PAHs are readily absorbed by fish and other aquatic organisms on exposure to contaminated materials attaining elevated level than those in the surrounding medium [81] with highest concentrations in sediment, followed by aquatic biota and lowest in water column. Bioaccumulation pattern of PAHs vary in aquatic organisms occupying different trophic levels [15] but existing is high biotransformative effect of the organisms that reduce the organic PAH physiological burden. Conversely, some of the Benz [a] pyrene concentrations considered most carcinogenic to human sourced from different locations and seasons were above safe margins stipulated by the Environment Canadain edible tissues of fish and /or shellfish for consumptions (0.004, 0.002, and 0.001 µg for low to high consumptions of 50, 100, and 200 g/week, respectively).Factorial effects of season and location were not observed in the current work, however, season has been known to affect the concentrations of PAH in aquatic ecosystem [3, 5, 16] and spatial location confirmed to play essential role in PAH level variations and distribution [3]. Regression modelling, principal component analysis (PCA) and positive matrix factorization (PMF) typically showed PAH concentrations in the environment to be higher during winter than summer and also impact the sources of the contaminant emissions in China [82, 83]. Trying to demonstrate geographical location effect on the concentrations of polycyclic aromatic hydrocarbons, Karyab et al. [3] maintain the PAH pollution of drinking water distribution system at sampled district culminated from proximity to and leaching of pipeline. Organic contamination of drinking water with PAHs has been earlier shown [11].Apart from the slight commercial activities, the available concentrations of the PAH could be hypothetically linked to atmospheric deposition as Anambra River is close to the heavily populated and industrially active city of Onitsha. Weather variables and wind dispersion could transport the substances and become deposited at nonimpact areas. Precipitation is a good climatic pathway of PAH input into the aquatic environment [84]. As hydrophobic organic substance, PAHs are rapidly sorbed to particles and attached to aquatic sediments on deposition [24, 85].The work provides fundamental reference point information on the water and fish PAH concentrations under the current level of anthropogenic activities at the Anambra River. Following the observations in the sampled eco-media, it appears that threats of PAH inputs currently exist for the Anambra River. However, we made emphases on the current consumption rates of fish in the area and established the daily intakes of PAHs among children, adolescent and adult based on field data. The levels of PAHs obtained in our studies relating to the daily human ingestions were lower than the values documented by earlier authors in Europe [47, 49-53]. The difference could be linked to the time of studies, number of PAHs analysed and level of industrialisations of the countries. Alternatively, probable explanation for the diversity in concentrations could be pointed to the number of food-groups and stuffs examined by the preceding studies. While they included all forms of food categories for total dietary exposure, our studies only looked at fish consumption as a single vehicle of exposure. The concentration values of PAHs obtained from cereals examined in Washington D.C., USA ranged from 0.040 to 0.060 µg/day [86] and are lower than present figures in the Anambra River. Public health risks from consuming aquatic food such as fish cannot be utterly excluded to be low. The continuous daily intake of fish is a potential health concern as long-term exposure to low acting concentrations is likely to activate biotoxicity, not considering temperature effects on PAH structural and media existential stability. In addition, mollusc and other invertebrates which form major items in local food menu readily accumulate high level of the contaminant since they are incapable of metabolising and excreting PAHs efficiently [87-89]. However, fish is not consumed fresh as they go through forms of heating prior to intake. The mechanisms of PAH formation and transformation (pyrolysis and pyrosynthesis) are dependent on heating temperature and time [90]. Consequently, the degradation could also be facilitated by low to high thermal process depending on the molecular structures [90-92]. The current findings did not cogitate the effect of heat on the PAH accumulations in fish due to heating or cooking before consumption but earlier studies acknowledged that heat-induced PAH degradations are apparently effective on low molecular ones leaving the abundant occurrence of high molecular compounds [92]. Cooking fish before eating can reduce the levels of some organic substances. One study investigated and showed the loss effect of polychlorinated biphenyl (PCB) congener’s levels in mackerel (Scomberscombrus) and herring (Clupeaharengus) during industrial hot smoking [93]. Similar studies have reported reduction of 20-70% lipophilic organic compounds PCBs and DDT in fish as a result of cooking [94, 95]. We cannot rule out basis on this fact the probability of PAH toxicity due to loss resulting from thermal processing of fresh fish before consumptions.Oil exploration, facility constructions, and potential exploitations are emerging threats to the environment of this river in the light of relative pollution and degradation being witnessed at the oil rich Niger Delta of Nigeria, where government concerns rest only on revenue generations with negligible effort to environmental management and sustainability. In other parts of Nigeria, PAHs have been also observed in principal media of freshwaters and food items relating to crude oil operations. Duke [21] detected considerable concentrations of PAH in Ekpan Creek of the Warri River, which were traced to crude oil pollution and petro-activities around the river. Food items have also been shown to be contaminated in the same region. Polycyclic aromatic hydrocarbons were detected in roasted food snacks prepared in Niger Delta [96]. Of particular concern is the aquatic health and biodiversity dynamics as latent oil spill and specific PAH components could unsympathetically impact them. Organisms at their early life stages (larvae and embryos) lack the physiological mechanistic systems of contaminant detoxification, homeostasis, and mobility in perception and response to localised environmental stress [63]. The cost to this early stage biodiversity would have detrimental significance on population structure of the resident organisms over time [97]. Polycyclic aromatic hydrocarbon exposure from crude oil has been demonstrated to induce anatomical and physiological anomalies in marine organisms [98-100].Nigeria uptil now has not come up with safe margins as standards for PAH level of food items rather depending on the mindless lifting of foreign safe limit values for the protection of the differential local ecosystems. In absence of occupational exposure and smoking, dietary intake has been pointed as an important exposure route for human [47, 101]. Fish has numerous dietary benefits to human, though exposures to toxic chemicals [102, 103] have been concerned issues for years.With increasing human exposure to environmental organic contaminant that are potentially carcinogenic such as PAHs, further studies are needed on different food-groups other than fish to delineate the extent of human dietary toxicant exposure. Continued monitoring of water quality conditions in the Anambra River needs to be effective and compared with this baseline data and other indicators to determine changes and variations in the aquatic health. Next phase of research is also expected on the PAH concentrations in sediments and on a wider scale to encompass other aquatic fauna and total flora of the threatened Anambra Basin ecosystem.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML