-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2014; 4(5): 234-237
doi:10.5923/j.re.20140405.04
Laboratory Evaluation of Limes against Invasive Swamp Eels, Monopterus albus Invading the Ifugao Rice Terraces, Philippines
Nancy Ann P. Gonzales
Ifugao State University, Lamut Ifugao, Philippines
Correspondence to: Nancy Ann P. Gonzales, Ifugao State University, Lamut Ifugao, Philippines.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Swamp eels, Monopterus albus have caused irreparably damaged rice fields in many parts of the Philippines. The study therefore sought to evaluate the effectiveness of shell limes on the control of swamp eels. The two-factorial Randomized Complete Block Design (RCBD) was used. Factor A included the types of shell lime and Factor B for the various levels of dosages. Sodium Cyanide was used as the control because it is the farmers’ practice. Analyses of variance (ANOVA) with SPSS were applied in the treatment of data. Results revealed that there is a significant difference on the effects of treatments. Sodium Cyanide kills M. albus at an average time of 15.20 minutes however it is a prohibited chemical. The use of 20 g Melanoides granifera shell lime: 500 ml of water or 20 g bivalve clam is also very effective in killing swamp eels at an average time of 60.73 minutes and 57.60 minutes respectively. The shell lime is organically produced, environment friendly and limes increase the fertility of the soil.
Keywords: Swamp eels, Shell lime, Rice terraces
Cite this paper: Nancy Ann P. Gonzales, Laboratory Evaluation of Limes against Invasive Swamp Eels, Monopterus albus Invading the Ifugao Rice Terraces, Philippines, Resources and Environment, Vol. 4 No. 5, 2014, pp. 234-237. doi: 10.5923/j.re.20140405.04.
1. Introduction
- Swamp eels or scientifically called Monopterus albus (Zuiew 1793) are commonly known as Asian swamps, rice-paddy and yellow eels. They belong to the sybranchidae fish family that grow as large as 100cm long and weigh as much as a pound. Their body colors range from olive to brown and occasionally light orange on the bottom. Some are colored yellow, black and gold spots as reported on various samples. They breath on air, travel on moist land and survive for weeks without food [3]. By burrowing in moist ground, they can survive for long period without water [1, 5]. As to food, they eat worms, frogs, tadpoles, shrimps, crayfish and other fishes [3].These swamp eels are nocturnal predators whose eggs are laid in a free-floating nest in shallow water. Male swamp eels guard the nest and young eels [5]. As to sex identity, all young Monopterus albus are females but some develop into males when they are adults and can change back to females should female densities are low. Change from one sex to another takes place a year and spawning is up to 1,000 eggs per female in a spawning event.Researchers and managers believe that dispersal of Monopterus albus may be controlled through a combination of electrical barriers, vegetation, removal and trapping. In Florida, agencies are collaborating on implementing an emergency response plan in an effort to eradicate the species or significantly reduce the population in areas adjacent to Everglades National Park to minimize environmental impacts caused by the Monopterus albus.Asian swamp eels are used as fish food [3]. Asians specifically Chinese immigrants are main consumers of swamp eels [5].In the Philippines, rice scientists are worried about Monopterus albus on their potentially damaging impact on rice fields. Farmers observed that these survive long period of drought by burrowing in the moist earth such as dikes and rice fields. The burrowed holes destroy the rice dikes affecting irrigation during the vegetative stage of rice resulting to water loss that affects nutrient management. Farmers first reported the rice paddy eel as a pest to the Bureau of Fisheries and Aquatice Resources (BFAR) in Tuguegarao, Cagayan two years ago complained that these swamp eels were eating fingerlings in fishponds. PhilRice declared then the rice paddy eels as “an indirect pest” during the last dry season of 2010 [4]. Rice farmers in some parts of Nueva Ecija and 2 other provinces reported that Monopterus albus appeared in their farms and damaged their irrigation dikes. Other PhilRice stations reported also the presence of Monopterus albus in rice farms in Isabela and Negros Occidental. Farmers used pesticide to control but few were found dead [6]. Farmers in Kiangan, Ifugao expressed alarm over the quick spread of these eels in their upland rice fields that are contributory to the destruction of the Ifugao Rice Terraces. The emergence of Monopterus albus as pest in their upland rice fields worsened the threat from giant earthworms which for years have been a headache for terraces farmers in Ifugao. Farmers from Ifugao said “we are calling on our officials to please help us find means how to get rid of these swamp eels because they pose bigger problems for us”.Objectives of the studyGenerally, it sought to manage the Monopterus albus pests with the use of environment friendly measures.Specifically, it aims at:1.Evaluating the effectiveness of limes in controlling Monopterus albus2. Determining the dosage of the lime that controls Monopterus albus3. Identifying if a significant difference exists on the effects of dosages in terms of limes 4. Identifying if there is a significant difference on the interaction effects of limes and dosages5. Determining if there exists a significant difference on the effects of lime in terms of the sizes of Monopterus albus.
2. Methodology
- The research was conducted at the laboratory of IFSU. The shell lime was prepared by burning empty shells and were powdered ready for application. The mixture of shell lime and water was prepared in a beaker following the desired dosages. For every mixture, one eel was placed and observed. The eel is considered dead if it exhibits no movement, the body follows any direction to which the eel is being moved and the body starts to harden.The use of shell lime natural product in controlling P. elongata [2] was the basis of the dosages for this study. Sodium cyanide was used as the control of the study because it is the chemical being used by the farmers in the place.Experimental DesignThe two-factorial in a Randomized Complete Block Design (RCBD) was used. Factor A holds the limes and factor B the dosages. The blocking factor is day trial.Factor A – Limes (ABCD)Factor B – Dosage (10 g, 20 g, 30 g)TreatmentsA1
 10 g Sodium cyanide, 500 ml waterA2
10 g Sodium cyanide, 500 ml waterA2 20 g Sodium cyanide, 500 ml waterA3
20 g Sodium cyanide, 500 ml waterA3 30 g Sodium cyanide, 500 ml waterB1
30 g Sodium cyanide, 500 ml waterB1 10 g Melanoides granifera lime: 500 ml waterB2
10 g Melanoides granifera lime: 500 ml waterB2 20 g Melanoides granifera lime: 500 ml waterB3
20 g Melanoides granifera lime: 500 ml waterB3 30 g Melanoides granifera lime: 500 ml waterC1
30 g Melanoides granifera lime: 500 ml waterC1 10 g Bivalve clam lime: 500 ml waterC2
10 g Bivalve clam lime: 500 ml waterC2 20 g Bivalve clam lime: 500 ml waterC3
20 g Bivalve clam lime: 500 ml waterC3 30 g Bivalve clam lime: 500 ml waterD1
30 g Bivalve clam lime: 500 ml waterD1 10 g Golden Apple Snail lime: 500 ml waterD2
10 g Golden Apple Snail lime: 500 ml waterD2 20 g Golden Apple Snail: 500 ml waterD3
20 g Golden Apple Snail: 500 ml waterD3 30 g Golden Apple Snail: 500 ml waterStatistical toolsANOVA was used to test the significant difference of the effectiveness of the limes, dosages, and interaction effects. To test for the mean comparison of significant difference, DMRT was employed. SAS was used in computing.
30 g Golden Apple Snail: 500 ml waterStatistical toolsANOVA was used to test the significant difference of the effectiveness of the limes, dosages, and interaction effects. To test for the mean comparison of significant difference, DMRT was employed. SAS was used in computing.3. Results
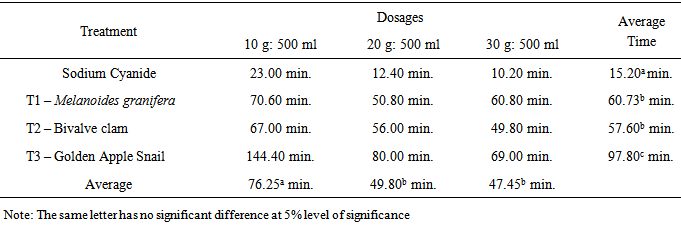
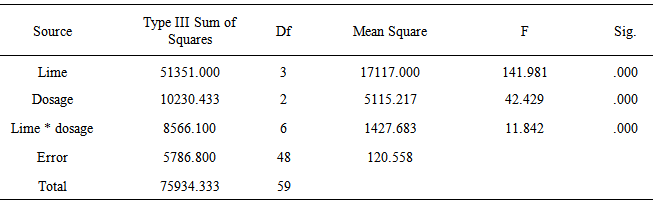
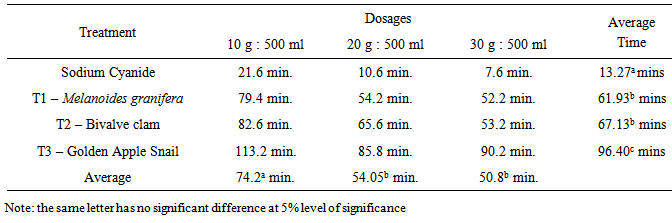
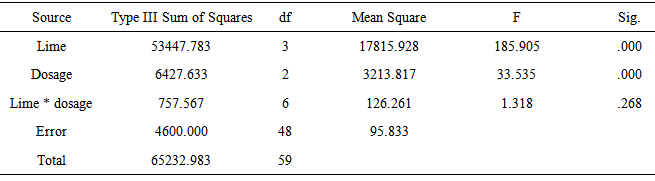
- Effects of Dosages and LimeTable 1 shows that sodium cyanide (alcampor) has an average killing time of 15.20 minutes across all the dosages of 10 g:500 ml, 20 g:500 ml and 30 g:500 ml for M. albus with lengths ranging from 25-35 cm. The Melanoides granifera shell lime mixtures killed the M. albus across dosages at an average time of 57-60 minutes. This means that the Melanoides granifera and bivalve clam take a longer time before the M. albus is killed. Both Melanoides granifera and bivalve clam have the same killing time. Golden Apple snail has the longest killing time with an average of 97.80 minutes. This shows that golden apple snail has a weaker strength in killing M. albus. The appropriate dosage to be used is 20 g of Melanoides granifera or bivalve clam: 500 ml water. It is better to use this dosage because lesser time is needed than to use 30 g lime:500 ml water where more lime is to be used and they have the same effects.
|
|
|
|
|
4. Conclusions
- 1. The various types of shell lime are effective in killing M. albus.2. The appropriate dosage that controls M. albus at the shortest time aside from the control is 20 g shell lime:500 ml water. The shell lime of Melanoides granifera or bivalve clam has the same killing time from the M. albus.3. There is significant difference on the effects of lime and dosage in controlling M. albus.4. There is significant interaction effect on the application of lime and the different dosages.5. There is no significant difference on the effects of lime as to the various sizes of M. albus.
ACKNOWLEDGMENTS
- The researcher acknowledges the Ifugao State University, Ifugao, Philippines for having funded this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML