-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2014; 4(5): 215-219
doi:10.5923/j.re.20140405.02
A Comparison of the Bacteriological Quality of Drinking Water for the Rural of Hooreh and Arjnk, Iran
Abdolmajid Fadaei
Department of Environmental Health Engineering, School of Health, Shahrekord University of Medical Sciences, Shahrekord, Iran
Correspondence to: Abdolmajid Fadaei, Department of Environmental Health Engineering, School of Health, Shahrekord University of Medical Sciences, Shahrekord, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Basically the life of human is related to safe water. The aims of this research was to investigatebacteriological quality of drinking water for the rural of Hooreh and Arjnk, Iran. In this cross-sectional descriptive study, a total of 72 water samples were taken from source water, storage reservoir and water distribution system in 2 villages. Total coliforms, Escherichia coli, and heterotrophic plate count (HPC) were enumerated. Statistical analysis of the results was performed using a SPSS 16 statistical software.The mean value and standard deviation for HPC bacteria/ml 1400±88 was the highest and the lowest 5±2 for source water and water distribution system, respectively. Based on obtained results 22.2% of the source water (river) samples were higher than 500HPC/ml. The correlation coefficient between HPC bacteria and turbidity, was 0.83, (P value < 0.05). High level of total coliform, E.coli and HPC bacteria were observed in surface water (river) compared to ground water (well). The free residual chlorine values of the storage reservoir and water distribution system are between 0.5 and 0.9 mg/l. There was not significant between level microbiological pollutants and seasons (P value>0.05). Poor sanitation of water supply is most causes of water contamination. It is recommended that sanitation measures are made to protect water resources from the contamination.
Keywords: Drinking water, Total coliform, Escherichia coli, Heterotrophic plate count
Cite this paper: Abdolmajid Fadaei, A Comparison of the Bacteriological Quality of Drinking Water for the Rural of Hooreh and Arjnk, Iran, Resources and Environment, Vol. 4 No. 5, 2014, pp. 215-219. doi: 10.5923/j.re.20140405.02.
Article Outline
1. Introduction
- Safe water supply is one of the main purposes in community. And development and improvement depend on healthy community. Bacteriological safety and quality of potable water is most important health concern to all people due to the potential of drinking water as carrier of microbial pathogens and cause of following disease in both developed and appearing economies of the world [1,2]. water pollution is most often due to human activities. The major ones are indiscriminate disposal of industrial, domestic and industrial sewage and agricultural and pasture runoff, municipal and domestic wastes in water channels, rivers, streams and lakes, etc [3, 4]. Bacterial contamination of drinking water is a main public health problems in the world, because this water can be an important vehicle of diarrheal diseases of infectious nature, which makes it necessary to evaluate the microbiological quality [4]. The health effects of exposure to disease-causing bacteria, viruses, and protozoa in drinking water are varied [5]. Bacteria (e.g., Vibrio, E. coli, Shigella and Campylobacter), viruses (e.g., polyomavirus, rotavirus, norovirus and hepatitis A) and protozoa (e.g., Toxoplasmosis gondii, Giardia and Cryptosporidium). The most common effects are gastrointestinal (nausea, vomiting, and diarrhoea) [6]. Other pathogens may infect the lungs, skin, eyes, central nervous system, or liver [7]. Verification of the microbial quality of drinking water includes testing for Escherichia coli (E. coli) as an indicator of fecal pollution. E. coli provides conclusive evidence of recent fecal pollution and should not be present in drinking-water. Another indicator were heterotrophs, heterotrophs are those microorganisms that use organic compounds for most or all of their carbon requirements. Most bacteria, including many of the bacteria associated with drinking water systems, are heterotrophs [8]. The two methods commonly used to detect coliforms in water include the multiple fermentation tube technique and the membrane filter technique [9]. Both WHO guideline and and Iranian standard values for bacteriological quality for drinking water suggested that fecal coliforms, total coliforms and Escherichia coli must not be detectable (nil) in any 100 ml sample. Some studies of bacteriological quality of drinking water for the rural areas had previously been conducted in world, for example, Trevett and colleagues reported from Honduran [10]. Amenu and colleagues reported from Ethiopia [11]. Amouei and colleagues reported from Mazandaran, Iran; Hoque and colleagues reported from Bangladesh [12, 13]. The purpose of this study is a comparison of the bacteriological quality of drinking water for the rural of Hooreh and Arjnk, Iran.
2. Materials and Methods
2.1. Study Area
- Shahrekord is the capital city of Chaharmahal and Bakhtiari Province, Iran. It is the largest city in the province.
2.2. Sampling
- A total of 72 samples were collected from source water, storage reservoir and water distribution system in Hooreh and Arjnk, Iran. This study was carried out in 2 villages of ShahreKord, Iran. Sources of drinking water Hooreh and Arjnk rurals were surface water (river) and ground water (well), respectively. Samples were collected from the source in 300 ml glass bottles sterile. Aseptic conditions and transported in coolers with ice in a temperature below 4ºc the Research Laboratory and Diagnostic Microbiology of the Shahrekord University of Medical Sciences to perform microbiological analysis.
2.3. Analytical Measurements
- Turbidity was measured by Nephelometer using 0.02 NTU standards. pH was measured using digital pH meter (Model Metrohm, pH Lab 827). Free residual chlorine was measured by a colorimeter kit.Towards evaluate the microbiological quality of the samples were analyzed for total coliform, coliform and heterotrophic plate count according to methodology described in the American Public Health Association guidelines [9]. Towards check the Most Probable Number (MPN) of total and faecal coliforms (E.coli) was used the technique of Multiple Tube Fermentation. The results were expressed as MPN/100ml. The heterotrophic bacteria count was performed using the pour plate technique, which were spread in 1 ml of the dilutions of 1 ml and 0.1 ml in sterile Petry plates and then added Plate Count Agar. These were incubated in bacteriological 36 ± 1℃ for 48 hours. The results were expressed as CFU / ml. The results were analyzed using the Statistical software SPSS and Excel.
3. Results and Discussion
3.1. Microbiological Quality of Water Arjnk Rural
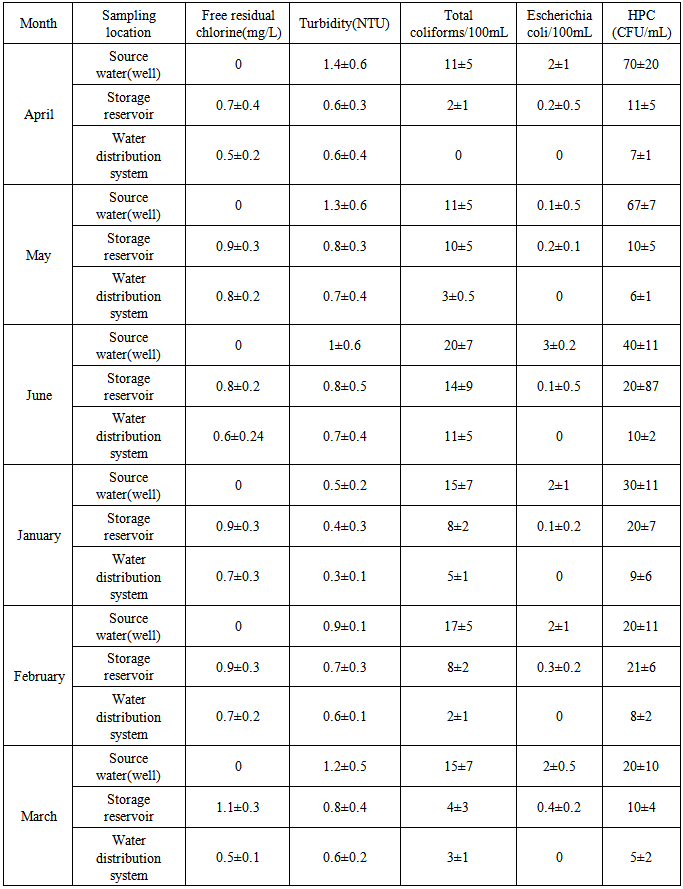
- According to the results of Table 1, Mean±SD score for HPC bacteria/ml 70±20 was the highest and the lowest 5±2 for source water (well) and water distribution system, respectively. Based on obtained results 100% of the source water (well) samples were lower than 500HPC/ml. Mean±SD score for total coliform/100ml 20±7 was the highest and the lowest 0 for 75±20 was the highest and the lowest 0.2±0.3 for source water (well) and water distribution system, respectively. The free residual chlorine values of the source water, storage reservoir and water distribution system are between 0.5 and 0.8 mg/l. Mean±SD score for turbidity (NTU) 1.4±0.6 was the highest and the lowest 0.3±0.1 for source water (well) and water distribution system, respectively.
|
3.2. Microbiological Quality of Water Hooreh Rural
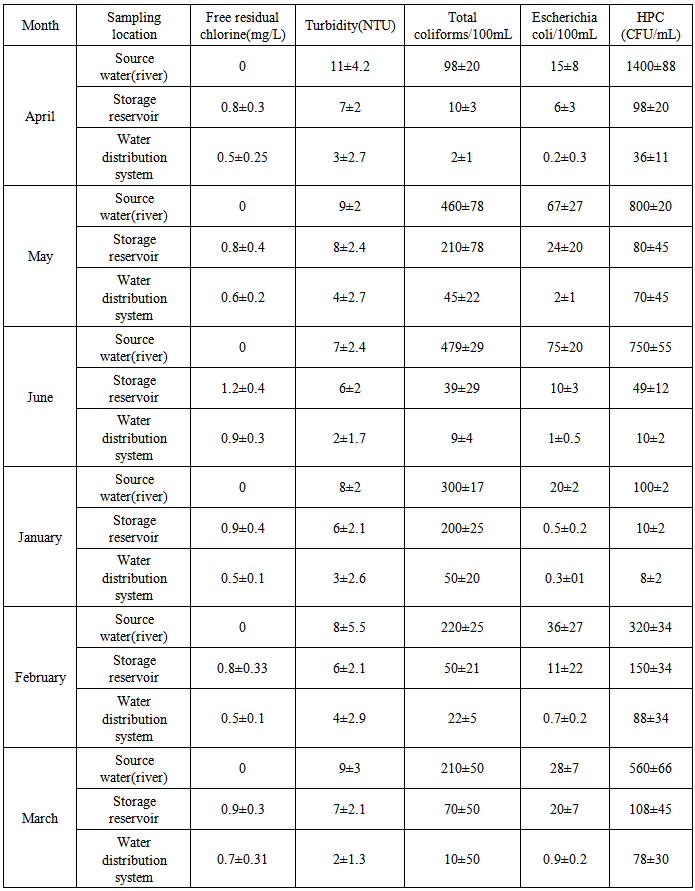
- According to the results of Table 2, Mean±SD score for HPC bacteria/ml 1400±88 was the highest and the lowest 8±2 for source water (river) and water distribution system, respectively. Based on obtained results 22.2% of the source water (river) samples were higher than 500HPC/ml. Mean±SD score for total coliform/100ml479±29 was the highest and the lowest 2±1 for source water (river) and water distribution system, respectively. Mean±SD score for E.coli/100ml 3±0.2was the highest and the lowest 0 for source water (river) and water distribution system, respectively. According to the Iran and WHO Guidelines for Drinking Water Quality, safe drinking water should not contain total coliforms, E. coli or any coliforms in a 100 ml water sample [14, 15].
|
4. Conclusions
- The contamination of these water sources with pathogenic organisms due to the absence of fencing of water sources that could prevent the entrance of animals, livestock grazing nearby water sources, people’s open area defecation, collecting of water with unclean containers, agricultural activities nearby water sources, defect of disinfection of the water reservoir, in some specially for surface water and unprotected well its difficulty to disinfect the water or treat the water sources before collecting the water. We would like to recommend the following important points: proper sanitary study, design and implementation of water and/or sanitation projects; regular disinfections, increased retention time of water in reservoirs is the most important factor of quality, the main reasons of breakdown. Maintenances and supervisions of water sources; and regular bacteriological assessment of all water sources for drinking should be planned and conducted.
ACKNOWLEDGEMENTS
- This research has been supported by Shahrekord University of Medical Sciences.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML