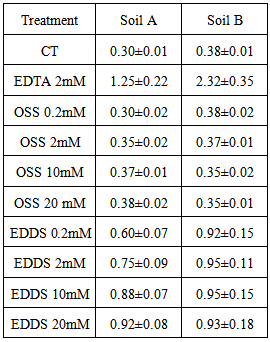
| [1] | Blaylock, M.J., D.E. Salt, S. Dushenkov, O. Zakharova, C. Gussman, Y. Kapulnik, B.D. Ensley, I. Raskin. 1997. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 31: 860–865. |
| [2] | Bucheli-Witschel, M., T. Egli. 2001. Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol. Lett. 25: 69-106. |
| [3] | Chen, Y., X. Li, Z. Shen. 2004. Leaching and uptake of heavy metals by ten different species of plants during an EDTA-assisted phytoextraction process. Chemosphere 57: 187–196. |
| [4] | do Nascimento, C.W.A. 2006b. Organic acids effects on desorption of heavy metals from a contaminated soil. Scientia Agricola 63: 276-280. |
| [5] | do Nascimento, C.W.A., D. Amarasiriwardena, B.S. Xing. 2006a. Comparison of natural organic acids and synthetic chelates at enhancing phytoextraction of metals from a multi-metal contaminated soil. Environmental Pollution 140: 114-123. |
| [6] | Doumett, S., D. Fibbi, E. Azzarello, S. Mancuso, S. Mugnai, G. Petruzzelli, M. Del Bubba. 2011. Influence of the Application Renewal of Glutamate and Tartrate on Cd, Cu, Pb and Zn Distribution Between Contaminated Soil and Paulownia Tomentosa in a Pilot-Scale Assisted Phytoremediation Study. Int. J. Phytoremediat. 13: 1-17. |
| [7] | Doumett, S., L. Lamperi, L. Checchini, E. Azzarello, S. Mugnai, S. Mancuso, G. Petruzzelli, M. Del Bubba. 2008. Heavy metal distribution between contaminated soil and Paulownia tomentosa, in a pilot-scale assisted phytoremediation study: Influence of different complexing agents. Chemosphere 72: 1481-1490. |
| [8] | Egli, T. 2001. Biodegradation of metal-complexing aminopolycarboxylic acids. J. Biosci. Bioeng. 92: 89-97. |
| [9] | Gee, G.W., J.W. Bauder. 1986. Particle-size analysis. In Klute, A. (Ed.) Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. Agronomy Monograph No. 9. 2nd edition. American Society of Agronomy/Soil Science Society of America, Madison. pp. 383–411. |
| [10] | Grčman, H., D. Vodnik, Š. Velikonja-Bolta, D. Leštan. 2003. Ethylenediaminedisuccinate as a new chelate for environmentally safe enhanced lead phytoextraction. J. Environ. Qual. 32: 500–506. |
| [11] | Jaworska, J.S., D. Schowanek, T.C.J. Feijtel. 1999. Environmental risk assessment for trisodium [S,S]-ethylene diamine disuccinate, a biodegradable chelator used in detergent applications. Chemosphere 38: 3597–3625. |
| [12] | Kos, B., D. Leštan. 2003. Induced phytoextraction/soil washing of lead using biodegradable chelate and permeable barriers. Environ. Sci. Technol. 37: 624–629. |
| [13] | Liu, D., W. Jiang, C. Liu, C. Xin, W. Hou. 2000. Uptake and accumulation of lead by roots, hypocotyls and shoots of Indian mustard [Brassica juncea (L.)]. Bioresource Technology 71: 273-277. |
| [14] | Luo, C., Z. Shen, X. Li. 2005. Enhanced phytoextraction of Cu, Pb, Zn and Cd with EDTA and EDDS. Chemosphere 59: 1-11. |
| [15] | Meers, E., A. Ruttens, M.J. Hopgood, D. Samson, F.M.G. Tack. 2005. Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 58: 1011–1022. |
| [16] | Meers, E., F.M.G. Tack, M.G. Verloo. 2008. Degradability of ethylenediaminedisuccinic acid (EDDS) in metal contaminated soils: Implications for its use soil remediation. Chemosphere 70: 358-363. |
| [17] | Mellem, J., H. Baijanth, B. Odhav. 2009. Translocation and accumulation of Cr, Hg, As, Pb, Cu and Ni by Amaranthus dubius (Amaranthaceae) from contaminated sites. J. Environ. Sci. Health 44: 568-575. |
| [18] | Meyers, D.E.R., G.J. Auchterlonie, R.I. Webb, B. Wood. 2008. Uptake and localisation of lead in the root system of Brassica juncea. Environmental Pollution 153:323-332. |
| [19] | Nelson, D.W., L.E. Sommers. 1996. Total carbon, organic carbon and organic matter. In Sparks, D.L. (Ed.) Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America Book Series. Soil Science Society of America Inc., Madison. pp. 961–1010. |
| [20] | Pedron, F., G. Petruzzelli, M. Barbafieri, E. Tassi. 2009. Strategies to use phytoextraction in very acidic soil contaminated by heavy metals. Chemosphere 75: 808–814. |
| [21] | Pedron, F., G. Petruzzelli, M. Barbafieri, E. Tassi. 2013. Remediation of a Mercury-Contaminated Industrial Soil Using Bioavailable Contaminant Stripping. Pedosphere 23: 104-110. |
| [22] | Pereira, B.F.F., C.A. de Abreu, U. Herpin, M.F. de Abreu, R.S. Berton. 2010. Phytoremediation of lead by jack beans on a Rhodic Hapludox amended with EDTA. Scientia Agricola 67: 308-318. |
| [23] | Petruzzelli, G., L. Lubrano, G. Guidi. 1989. Uptake by corn and chemical extractability of heavy metals from a four year compost treated soil. Plant Soil 116: 23–27. |
| [24] | Quartacci, M.F., A. Argilla, A.J.M. Baker, F. Navari-Izzo. 2006. Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 63: 918-925. |
| [25] | Quartacci, M.F., A.J.M. Baker, F. Navari-Izzo. 2005. Nitrilotriacetate- and citric acid-assisted phytoextraction of cadmium by Indian mustard (Brassica juncea (L.) Czernj, Brassicaceae). Chemosphere 59: 1249–1255. |
| [26] | Souza, L.A., F.A. Piotto, R.C. Nogueirol, R.A. Azevedo. 2013. Use of non-hyperaccumulator plant species for the phytoextraction of heavy metals using chelating agents. Sci. Agric. 70: 290-295. |
| [27] | Sumner, M.E., W.P. Miller. 1996. Cation exchange capacity and exchange coefficients. In Sparks, D.L. (Ed.) Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America Book Series. Soil Science Society of America Inc., Madison. pp. 1201–1230. |
| [28] | Sun, B., F.J. Zhao, E. Lombi, S.P. McGrath. 2001. Leaching of heavy metals from contaminated soils using EDTA. Environ. Pollut. 113: 111–120. |
| [29] | Tandy, S., K. Bossart, R. Mueller, J. Ritschel, L. Hauser, R. Schulin, B. Nowack. 2004. Extraction of heavy metals from soils using biodegradable chelating agents. Environ. Sci. Technol. 38: 937–944. |
| [30] | Thomas, G.W. 1996. Soil pH and soil acidity. In Sparks, D.L. (Ed.) Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America Book Series. Soil Science Society of America Inc., Madison. pp. 475–490. |
| [31] | Wu, L.H., Y.M. Luo, X.R. Xing, P. Christie. 2004. EDTA-enhanced phytoremediation of heavy metal contaminated soil with Indian mustard and associated potential leaching risk. Agriculture, Ecosystems and Environment 102: 307–318. |

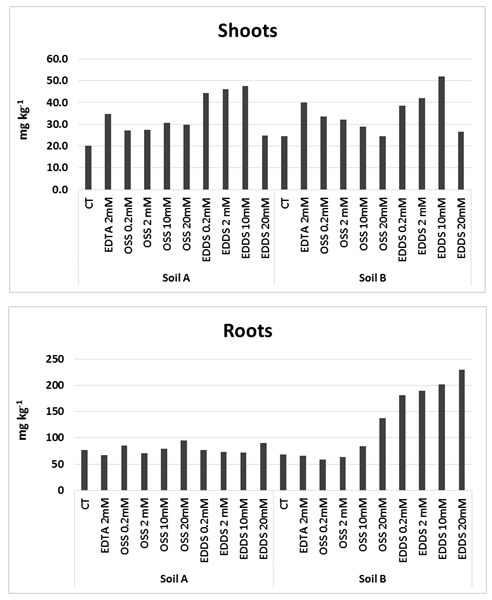
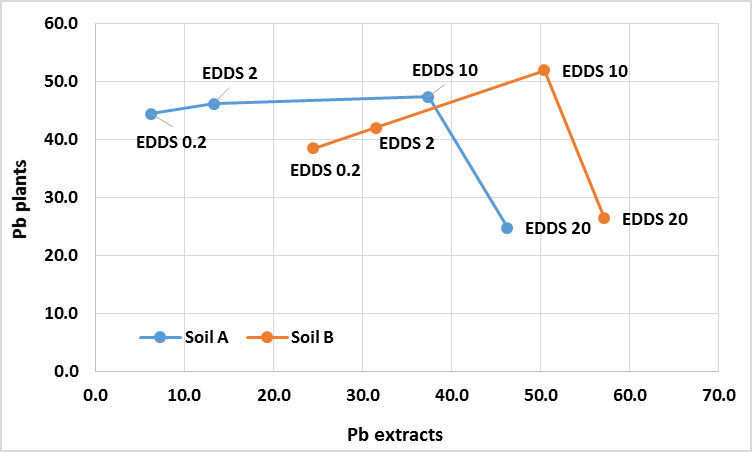
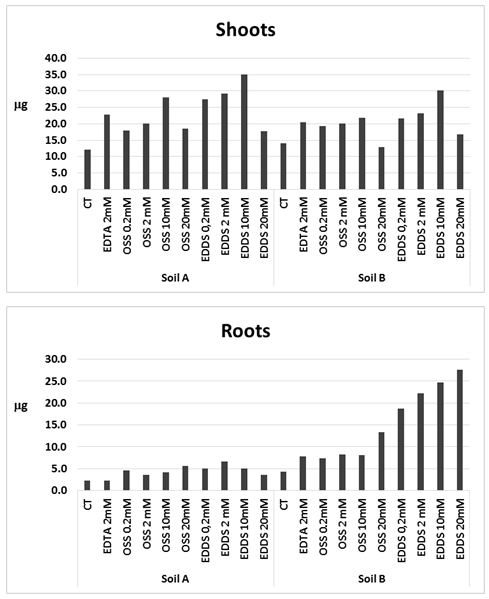
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML