-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2014; 4(2): 95-103
doi:10.5923/j.re.20140402.03
Diversity of Macrobenthos Communities and Their Relationships with Environmental Factors in Jajroud River, Iran
Niusha Amri 1, Shahla Jamili 2, Soudabe Abdolbaghian 1
1Science and Research Branch of Islamic Azad University, Tehran, Iran
2Iranian Fisheries Research Organization, Tehran, Iran
Correspondence to: Niusha Amri , Science and Research Branch of Islamic Azad University, Tehran, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This paper evaluates the diversity of common groups of macrobenthos include: Diptera, Ephemeroptera, Odonata, Plecoptera, and Gastropoda and their relationships with environmental variables in the Jajroud river northeast of Tehran province (capital of Iran). The work has been extended out over a period of 12 months through three stations. Macrobenthos sampling has been carried out monthly with three replications in each station. Water temperature, dissolved oxygen, pH, BOD, phosphate and nitrate concentrations were within ranges usually capable of backing up a diverse biota. The other components such as water depth, water width, water flow and contamination were as well taken. The methodologies applied by a number of research groups were generally similar, making it possible to compare outcomes between different areas. Three metrics were utilized to show the diversity: Margalef’s richness, Shannon-Weaver's diversity index (Ĥ) and evenness index (E). From108 Surber samples, 29 families, 39 genus and 92 species were identified (42% Ephemeroptera, 38% Diptera, 18% Plecoptera, 1.2% Gastropada and 0.8% Odonata of the total fauna population). Chironomidae, Baetidae and Perlodidae were the most dominant families. The biodiversity of the community of the upper reaches of the river (ST1) was the highest that of the lower reaches (ST2) was the lowest that of (ST3) ranked in the middle. In this work, the presence of pollution-tolerant families in (ST3) indicates that this site is ecologically unhealthy. The diversity of Gastropoda and Chironomidae were significantly affected by pollution (positively) and water depth; whereas the diversity of Ephemeroptera by the dissolved oxygen and water temperature respectively. Besides the diversity of Odonata was affected by stream width. The outcome of a One-Way ANOVA for three metrics indicates the P - value and F-critical 3.1 and 5.1 respectively.
Keywords: Diversity, Richness, Evenness, Macrobenthos and Jajroud River, Iran
Cite this paper: Niusha Amri , Shahla Jamili , Soudabe Abdolbaghian , Diversity of Macrobenthos Communities and Their Relationships with Environmental Factors in Jajroud River, Iran, Resources and Environment, Vol. 4 No. 2, 2014, pp. 95-103. doi: 10.5923/j.re.20140402.03.
1. Introduction
- Macrobenthic community plays an significant part in aquatic ecosystems as primary and secondary consumers as easily as in biological assessments [1]. A detailed and perfect knowledge of the bottom fauna is not merely important for the purpose of productivity [2] but is also helpful in seeing the diversity of the habitat. Sustaining biological diversity is a priority for nature conservation in terrestrial, maritime, and freshwater environments [3]. Thus, the assessment of biological diversity in freshwaters plays a really important role as the basis for nature protection.Diversity, distribution and abundance of macrobenthos depend on the characteristics of their environment such as pollution condition, organic matter, content, soil texture and sediment [4, 5]. Because they alter in their adjustment to environmental conditions and their tolerance of or sensitivity to contamination, the parameters of benthic animals (such as their community structure, dominant species, variety and abundance) can be utilized to reflect environmental quality [6].In the other words, by keeping the changes in the number of organism types in the creek system, scientists can see the biological population richness and also by calculating the number of organisms in surface unit or their frequency, the influences of human beings on the river can be seen. Conditions invertebrate benthos population is a observation of larger aquatic food network variety and stability [7].Typically, the diversity indices used in biological assessment studies are calculated for highly important indicator groups like Chironomidae [8] and EPT (larval Ephemeroptera, Plecoptera, andd Trichoptera) or for a selected part of macrobenthictaxi [9], identifiedatn the level of genus or famil [10], and muchmore rarey for all macrobenthos [11].The assumption that habitat degradation results in a substantial and predictable decrease in taxonomic diversity is an important aim of several methods of biological assessment based on fresh water organisms, especially on benthic invertebrates [12]. The decrease in taxonomic diversity due to habitat degradation could be measured as a reduced species richness (stressors not allowing less tolerant species to colonize or to run in degraded sites [13] or depletion in the values of any of numerous indices of diversity (stress eliminates sensitive taxes and consequences in a greater numerical dominance of those able to persist [14]). Moreover, diversity indices respond strongly and unpredictably when a taxonomic detail changes [14], so it can also make the search for general ecological determinants of stream taxonomic diversity not-doable. Thither are many sharp or subtle differences in response of each higher taxon of stream macrobenthos to important environmental components. This variety of differences between higher taxes in taxonomic diversity has been shown in numerous fields. The diversity of Odonata seems to be strongly correlated with the local climatic specifics [15]; moreover, it is more sensitive than the diversity of other taxonomic groups to the social system of riparian vegetation [16]. The profusion of four insect orders studied by Rosemond et al. (1992) was affected in different ways by chemical parameters of stream waters [17].Moreover, the kinship between the benthic community structure and environmental variables has been the topic of numerous investigations [18, 19, 20, 21, and 22]. Poff and Ward [23] identified stream flow variability as a major factor affecting other abiotic and biotic elements that regulate lotic macrobenthic patterns.In this work, the researcher evaluated diversity of the chosen groups of macrobenthos, analyzed their relationships with environmental components and also compared the reaction of different groups to environmental variables.
2. Material and Method
- This work has been borne out in Jajroud River -that is located in 50˚ 51' N to 50˚ 55' N of longitude and 34˚ 55' E to 35˚ 05' E of latitude -over a period of a year and by month sample-taking. Jajroud River is situated 30 km North-East of Tehran (capital of Iran) and flows from its origin (Alborz Mountains) to the lower altitude. Many villages were irrigated by this river. Its basin has an area of 710 km². Referable to the mountainous area and the problem of waste disposal, all the wastewater of villages enters to the river. Water coming from the Latyan dam supplies drinking water to approximately two million six hundred thousand people in East Tehran. Thus, it is necessary to protect water quality.At the origin, by various quality-based sample taking, three places were picked out along the river in three regions at the top, in the center, and at the rear end of the river, according to the texture of the riverside and difference in biological, chemical, and physical agents.. ST1 (Garmabdareh) was selected as a reference site was primarily vegetated with relatively mature native forest, short or no residential development, and no permitted discharges (coal mining, petroleum/gas extraction, or sewage treatment plants). ST2 (Meygoon) and ST3 (Roodak) were Non-reference sites, or test sites with span a range of observed human impacts to the watershed, stream, or individual reach (Fig.1).
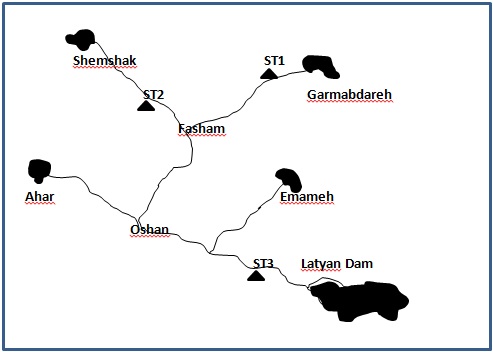 | Figure 1. Jajroud River map and general location of stations |
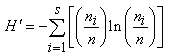 | (1) |
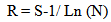 | (2) |
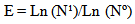 | (3) |
3. Results
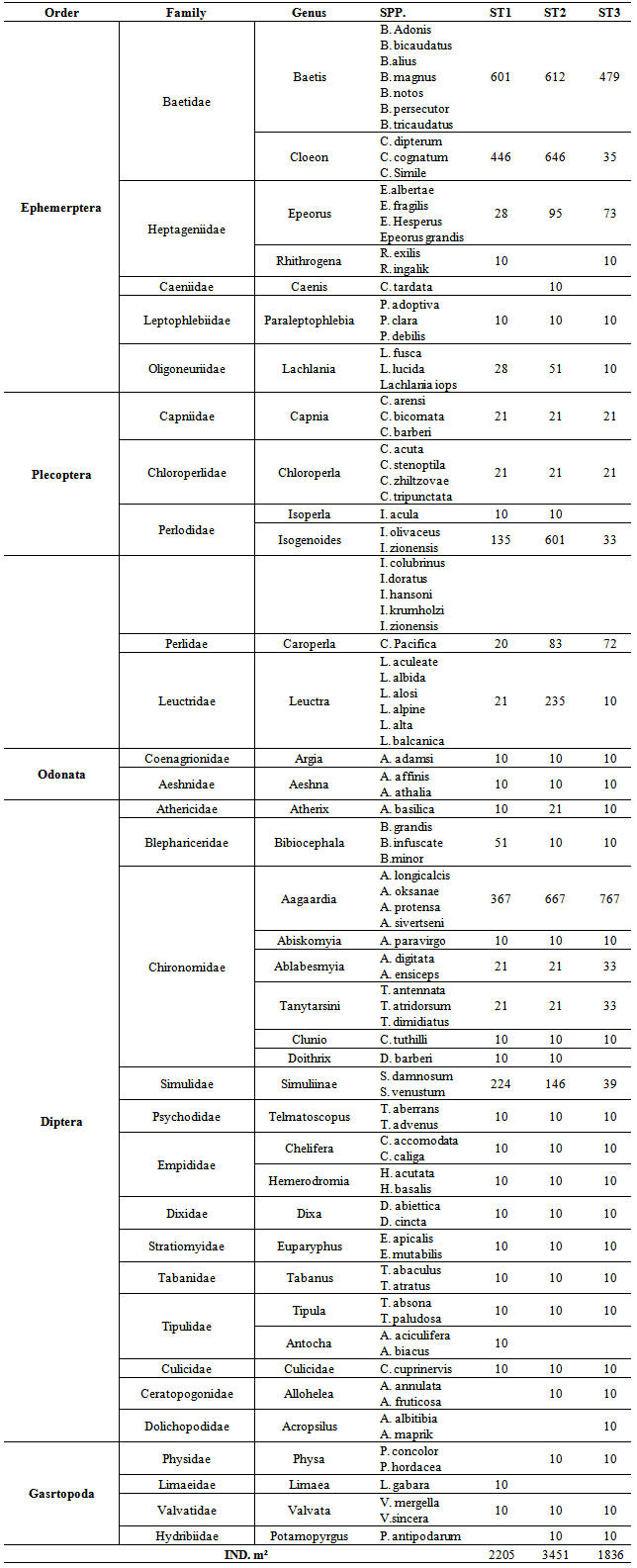
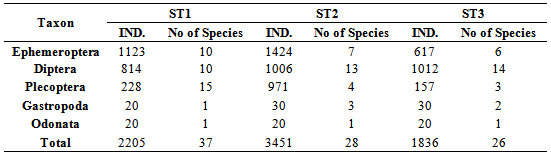
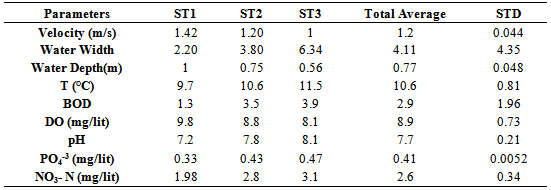
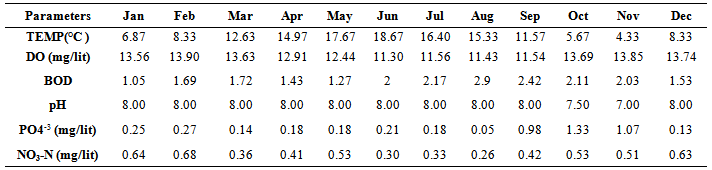
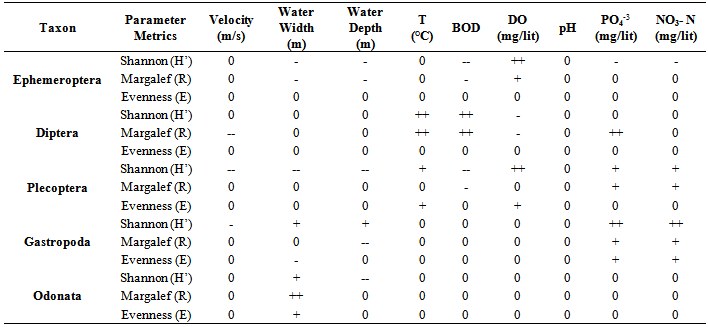
- Location & time results of physicochemical parameters as well as diversity indices are shown at a lower place:The ranges of the physicochemical and hydrological parameters in Jajroud Stream are listed in Table5 and 6. Mean water velocity was 120 cm. s-1 in a Jajroud stream with a minimum of 100 cm. s-1 in ST3 and a maximum of 142cm. s-1 in ST1. Water depth rarely exceeded 75 cm.The minimum and maximum water temperature recorded was 4ºC during November and 18.7ºC during June respectively, with an average of 11.52ºC. Minimum dissolved oxygen concentration, 11.3 mg.l-1, was recorded during June and maximum dissolved oxygen concentration, 13.9 mg.l-1 occurred during February. Dissolved oxygen values were above saturation and BOD5 was low at all times at all sites. The differences in dissolved oxygen concentrations and BOD5 between the stations were not significant (p > 0.05). The amount of NO3-N ranged from 0.26 mg. l-1 to 0.68 mg. l-1 and PO4-P ranged from 0.13 mg. l-1 to 1.33 mg. l-1 during the study period. The PO4-P values were generally low at all sites. NO3-N and PO4-P concentrations increased from upstream sites toward downstream. The use of agricultural fertilizers and urban sewage are believed to increase the Nitrate Nitrogen.A total of 92 macrobenthic fauna species represented by five diverse groups were encountered, of which Diptera were the most dominant group with 38 species and contributed numerically up 38% of the population. Ephemerptera consist of 23 species and contribute to42% of the total fauna population. Plecoptera consist of 15 species and contribute to18% of the total fauna population. Gastropoda consist of 6 species and contribute to 1.2% of the total fauna population. Also, Odonata include 3 species and contribute to0.8% of the total fauna population (Table 2, 3).The Shannon-Wiener index (H) ranges between 1.33 (station 2, winter) and 2.25 (station 1, summer). The evenness component (E) varied from 0.40 (station2, winter) to 0.60 (station 1, summer). The richness component (R) ranged between 5.40 (station 2, winter) to 5.85 (station1, summer) (Table 4).The seasonal changes of the benthic family abundance tended to increase from fall-winter to spring. The benthic diversity also had an increasing trend from stations 3 to 1 (downstream to upstream).The correlations between diversity of the analyzed benthic fauna and the values of environmental parameters were highly divergent. It did not correlate with pH in all three sites. The diversity of Gastropoda increased with the increasing of pollution, concentration of PO4-3 and NO3- N (p <0.01), water width and water depth (0.01 <p <0.05), and decreased with water velocity (0.01 <p <0.05). In the second group, the diversity of Chironomidae was correlated with the BOD and water temperature (p <0.01). The diversity of larval Odonata, being a third group, was higher in wider streams and lower when the water depth increased (Table 7).
|
|
|
|
|
|
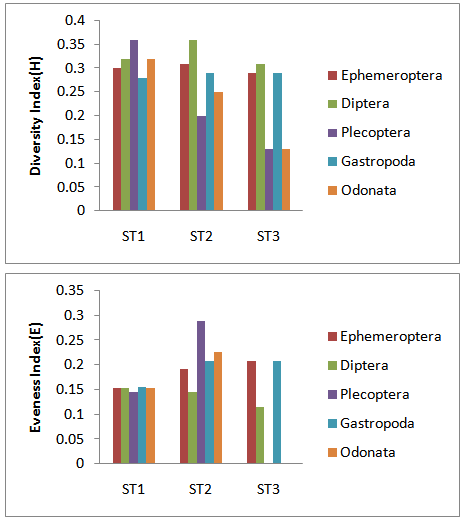 | Figure 2. Value of Shannon-Weaver index of diversity (upper plot) and values of evenness index(lower plot) for chosen taxa sampled in the Jajroud river. Value of indces estimated for 1000 individuals |
4. Discussion
- One of the main goals of benthic ecology has been to understand the mechanisms regulating relationships between physicochemical parameter and organisms [33-36]. The present study shows that the macrofaunal communities characterized by temporal and spatial changes in its population and distribution pattern seems to be fully governed by the physicochemical and hydrobiological characteristics of the environment.Richer communities have been found in station I. The macrobenthic faunal density ranges from 10 to767 IND/m-2 in all the stations. This density is lower than the macrobenthic faunal densities as reported by Parulekar AH [37] for Zuari estuary (50 to 1,437 in/m-2), by Parulekar AH and Ansari ZA [38] for Andaman seas (80 to 998 in/m-2), and comparable with that of Harikantra SN et al. [39] who record the density range of macrobenthos from 50 to 3,715 ind/m-2 in the shelf region along the west coast of India. The difference in the benthic macrofaunal densities of different aquatic systems could be attributed mainly to variations in substratum, sediment organic matter level and predation.The species composition of benthic macrofauna in the present observation shows the dominance of Diptera followed by Ephemeroptera and Plecoptera. The similar preponderance of Diptera has been observed earlier by Sankar G [40] in Muthupet lagoon, Sunil Kumar [41] in Cochin backwaters, Prabha Devi L [42] in the Coleroon estuary, and Ansari ZA et al. [43] in Mandovi estuary. The benthic population density shows seasonal variation in such a way that the maximum is recorded in summer and the minimum during winter at all the stations.While moderate pollution significantly declined diversity of ephemeropterans in the streams under present study (Tab. 3), which is congruent with the results of numerous other studies [44] the diversity of Gastropoda and Diptera is visibly high. They have a more opportunistic bearing potential ability to colonize in stressed environments [45]. The aforementioned adaptable nature of Chironomides may be a plausible reason for their dominance in the species composition and their abundance in the present investigation. In the present study, Ephemeroptera from the second dominant group followed by Diptera.During the study, dissolved oxygen was high during the winter season at all sites, which might be due to the cumulative effect of higher wind velocity coupled with heavy rainfall and the resultant freshwater mixing. Relatively lower values were observed during summer; this may be due to the increased surface water temperature, which reduces the dissolution of O2 in the surface waters. It is well known that temperature affects the dissolution of oxygen [46]. Hydrogen ion concentration (pH) in surface waters remained alkaline at all sites throughout the study period with the minimum value during October and November. However, the present study did not find a characteristic relationship between pH and temperature and macrobenthic fauna, confirming that the fauna of independent freshwater system requires specific environmental characters. Organic nutrients enhance the growth of different types of algae that provide food resources for benthos [47]. In the present study, the higher density macrobenthos is observed during summer season at St2. Higher organic matter gets deposited during the spring season and it would be converted into available food by various fungal and bacterial sources, which in turn increase the macrobenthic forms especially Chironomidaes [48].The lower species diversity was recorded during winter in St2 and higher diversity values during the summer in the St1. This is in conformity with the earlier observations made in Vellar [49] and Coleroon estuaries [50]. Moreover, Pearson TH and Rosenberg R [51] proposed that the use of diversity indices is advantageous for the description of fauna at different stages in succession. The species richness of benthic macrofauna was found maximum during the summer season in St1 (5.85). A similar observation was reported by Kumar RS [52] in Cochin backwaters. The low richness was recorded during winter in St2 (4.40) might be due to the high freshwater inflow which in turn affect the distribution of benthos, particularly the Chironomidaes. Maximum diversity and richness recorded during summer at the all sites might be due to stability and optimum environmental factor. Shannon diversity was moderate and it was in the range of 2.25-1.33. The minimum (1.33) and maximum (2.25) was recorded at station II during the winter season and at the station I during the summer season, respectively.From the present study, it could be concluded that the hydrography nutrients and dissolved oxygen are the major factors responsible for fluctuation in benthic macrofaunal assemblages in the study area.Additionally, the diversity correlated positively with spring stability and productivity, and negatively with altitude. According to Jacobsen (2008) [53], the negative correlation of stream-benthos diversity with altitude reflects the oxygen concentration in water. Stability of abiotic parameters and isolation are important and strong determinants of the diversity of benthos, especially in ponds and streams [54].It seems that differential abundance in Jajroud stream is due to naturally-occurring variations in hydrological and ecological properties such as low stream order, proper velocity (in the range of 30 to 120 cm. s-1 and flow condition.The middle and lower reaches of major inflowing rivers are most influenced by human activities and consequently have the worst water quality. Lower diversity and richness values in St2 indicate stressful environment for benthic fauna. This situation emphasizes the importance of formulating and implementing a management program regarding the control of human activities development in the future, of course within a framework of an integrated management plan for the whole of its sub-river basin.Moreover, St3 has been severely polluted because of water shortage, the discharge of industrial wastewater and mostly untreated municipal sewage. Increasing of Chironomide species, especially tolerant groups in this station confirmed this fact.The upper reaches of the inflowing rivers, especially in the mountains where human influence was relatively weak had the best water quality and consequently the greatest values for species diversity, species evenness and species richness indices.
ACKNOWLEDGEMENTS
- The authors express their gratitude and thanks to Dr. Shala Jamili, Iranian Fisheries Research Organization, Science and Research Branch of Islamic Azad University, for providing the needed facilities.
References
| [1] | Popchenko, V.I., 1971. Consumption of oligochaeta by fish and invertebrates. J. Ichthyol., 11: 75-80. |
| [2] | Ramkumar, R., J.K.P. Edward and M. Jaikumar, 2010. Macrobenthic community structure on tuticorin coastal water, Gulf of Mannar, Southeast coast of India, World J. Fish and Marine Sci., 2 (1): 70-77. |
| [3] | Brooks, T.M., Mittermeier, R.A., da Fonseca, G.A.B., Gerlach, J., Hoffmann, M., Lamoreux, J.F., Mittermeier, C.G., Pilgrim, J.D., and Rodrigues, A.S.L. 2006. Global biodiversity conservation priorities. Science, 313 (5783): 58–61. doi:10.1126/science.1127609. |
| [4] | Dahanayakar, D.D.G.L. And M.J.S. Wijeyaratne, 2006. Diversity of macrobenthic community in the Negombo estuary, Srilanka, with special reference to environmental conditions. Srilanka, J. Aguat. SCI., 11: 43-61. |
| [5] | Perkins, E.J., 1974. The biology of estuarine and coastal waters, Academic Press, London, pp.: 678. |
| [6] | Gao. F (2011).Ecological Characteristics of Macrobenthic Communities in the Chaohu Lake Basin and Their Relationship with Environmental Factors. |
| [7] | Likens, G.E., 2010. River Ecosystem Ecology. Academic Press, UK. ISBN: 9780123819994, PP 81-212, 410. |
| [8] | Cranston, P.S. 1995. Introduction. In: Armitage, P., P.S. Cranston & L.C.V. Pinder (Eds), the Chironomidae. The biology and ecology of non-biting midges. Chapman & Hall, London: 1-7. |
| [9] | Barbour, M.T.J., G.E. Gerritsen, R. Griffith, E. Frydenborg, J.S. McCarron, M.L. White & A. Bastian. 1996. Framework of Biological Criteria for Florida Streams Using Benthic Macroinvertebrates. J. N. Am. Benthol. Soc., 15:185-211. |
| [10] | Fleituch, T., H. Soszka, D. Kudelska & A. Kownacki. 2002. Macroinvertebrates as indicators of water quality in rivers: a scientific basis for Polish standard method. Arch. Hydrobiol. Suppl. 141/3-4: 225-239 |
| [11] | Johnson, R.K. & D. Hering. 2009. Response of river inhabiting organism groups to gradients in nutrient enrichment and habitat physiography. J. Appl. Ecol., 46: 175-186. |
| [12] | Lenat, D.R. 1988. Water quality assessment of streams using a qualitative collection method for benthic macroinvertebrates. J. N. Am. Benthol. Soc., 7: 222-233. |
| [13] | Townsend, C.R. & A.G. Hildrew. 1994. Species traits in relation to a habitat template for river systems. Freshwater. Biol.31: 265-275. |
| [14] | Jones, F.C. 2008. Taxonomic sufficiency: the influence of taxonomic resolution on freshwater bio assessments using benthic macroinvertebrates. Environ. Rev., 16: 45-69. |
| [15] | Eversham, B.C. & J.M. Cooper. 1998. Dragonfly Sp ciesrichness and temperature: national patterns and latitutetrends in Britain. Odonatologica, 27: 287-414 |
| [16] | Smith, J., M.J Samways & S. Taylor. 2007. Assessing riparian quality using two complementary sets of bio indicators. Biodiv. Conserv., 16: 2695-2713. |
| [17] | Rosemond, A.D., S.R. Reice, J.W. Elwood & P.J. Mulholland. 1992. The effects of stream acidity on benthic invertebrate communities in the south-eastern United States. Freshwat. Ecol., 27: 193-209. |
| [18] | Strickland JDH, Parsons TR (1972) A Practical Handbook of Seawater Analysis. Fish Res Board Canada, 310. |
| [19] | Fauvel P (1953) the fauna of India including Pakistan, Ceylon, Burma and Malaya, Annelida Polychaeta. Allahabad: The Indian Press. |
| [20] | Day JH (1967) A Monograph on the Polychaeta of Southern Africa, Part I (Errantia) & II (Sedentaria). London: Trustees of the British Museum (Natural History). |
| [21] | Subba Rao NV, Surya Rao KV, Maitra S (1991) Marine mollusks, State Fauna Series, Fauna of Orissa, Kolkata. Zool Sur India 1: 1-175. |
| [22] | Koperski, P. 2010. Diversity of macrobenthos in lowland streams: ecological determinants and taxonomic specificity. J. Limnol.69: 1–14. Doi: 10.3274/JL10-69-1-08. |
| [23] | Gray, J (1974) Animal-sediment relationships. Oceanog Mar Biol Annul Rev 12: 223-261. |
| [24] | Greenberg, A.E., Clesceri, L.S. & Eaton, A.D (Eds.), 1992. Standard Methods for the examination of water and wastewater, American Public Health Association, Washington. D.C. |
| [25] | Marshall, J.C., A.L. Steward & B.D. Harch. 2006. Taxonomic resolution and quantification of freshwater macroinvertebrate samples from an Australian dryland river: the benefits and costs of using species abundance data. Hydrobiologia, 572: 171-194. |
| [26] | Warwick, R.M. and K.R. Clarke, 1993. Comparing the severity of disturbance: ameta-analysis of Marin macrobenthic community data, Marine Ecology Progress Series, 92: 221-231. |
| [27] | Klink, A.G. & H.K.M. Moller-Pillot. 2003. Chironomidae larvae. Key to higher taxa and species of the lowlands of Northwestern Europe. World Biodiversity Database, ETI. |
| [28] | Wiederholm, T. 1983. Chironomidae of the Holarctic region. Keys and diagnoses. Part 1. Larvae. Entomol. Scand.Suppl. 19: 1-457. |
| [29] | Piechocki, A. 1979. Mięczaki (Mollusca); ślimaki (Gastropoda). Fauna słodkowodna Polski. 7. PWN, Warszawa. |
| [30] | Nesemann, H. & E. Neubert. 1999. Annelida, Clitellata: Branchiobdellida, Acanthobdellea, Hirudinea. Süßwasserfaunavon Mitteleuropa 6/2, Spectrum, Heidelberg. |
| [31] | Eiseler, B. 2005. Bestimmungsschlüssel für die Eintagsfliegenlarven der deutschen Mittelgebirge und des Tieflandes.Lauterbornia, 53: 1-112. |
| [32] | Heidemann, H. & R. Seidenbusch. 2002. Die Libellenlarven Deutschlands. Tierwelt Deutschlands, 72. Goecke & Evers Verlag, Keltern: 328 pp. |
| [33] | Gray J (1974) Animal-sediment relationships. Oceanog Mar Biol Annul Rev 12: 223-261. |
| [34] | Rhoads D (1974) Organism-sediment relations on the muddy sea floor. Oceanog Mar Biol 12: 263-300. |
| [35] | Snelgrove PVR, Butman CA (1994) Animal Sediment Relationships Revisited, Cause versus Effect. Oceanog Mar Biol 32: 111-177. |
| [36] | Aller JY, Woodin SA, Aller RC (2001) Organism Sediment Interactions. Columbia: University of South Carolina Press. |
| [37] | Raveenthiranath N (1990) Ecology of Macrobenthos in and around the Mahandrapalli Region of Coleroon Estuary, Southeast coast of India. Ph.D. thesis, Annamalai University, India, 231. |
| [38] | Parulekar AH, Ansari ZA (1986) Spatial and temporal changes in benthic macrofauna from Mandovi & zuari estuaries of Goa, West coast of India. Indian J Mar Sci 15: 223-229. |
| [39] | Harikantra SN, Nair A, Ansari ZA, Parulekar AH (1980) Benthos of Shelf Region along the West Coast of India. Indian J Mar Pollu Bull 14: 41-46. |
| [40] | Sankar G (1998) Studies on the Hydrobiology, Benthic Ecology and Fisheries of Muthupet Lagoon. Ph.D. Thesis, Annamalai University, India, 105. |
| [41] | Sunil Kumar (1995) Macrobenthos in mangrove ecosystem of Cochin backwater, Kerala (South west coast of India). Indian J Mar Sci 24: 56-61. |
| [42] | Prabha Devi L (1994) Ecology of Coleroon estuary, Studies on Benthic Fauna. J Mar Biol Ass India 36: 260-266. |
| [43] | Ansari ZA, Ingole BS, Banerjee, G, Parulekar AH (1986) Spatial and Temporal Changes in Benthic Macrofauna From Mandovi & Zuari Estuaries of Goa, West coast of India. Indian J Mar Sci 15: 223-229. |
| [44] | Fleituch, T., H. Soszka, D. Kudelska & A. Kownacki. 2002. Macroinvertebrates as indicators of water quality in rivers:a scientific basis for Polish standard method. Arch. Hydrobiol. Suppl. 141/3-4: 225-239 |
| [45] | Raveenthiranath N (1990) Ecology of Macrobenthos in and around the Mahandrapalli Region of Coleroon Estuary, Southeast coast of India. Ph.D. thesis, Annamalai University, India, 231. |
| [46] | Viijayakumar S, Rajesh KM, Mridula RM, Hariharan, V (2000) Seasonal Distribution And Behavior of Nutrients With Reference to Tidal Rhythm in the Mulki estuary, Southwest coast of India. J Mar Biol Ass India 42: 21-31. |
| [47] | Hart CL, Odum EP (1970) Mechanisms Maintaining High Productivity in Georgia estuaries. Proceed Gulf Caribbean Fish Ins 14th Annul Ses 75-80. |
| [48] | Sunilkumar R, Antony A (1994) Preliminary Studies on the Polychaete Fauna of the Mangrove Areas of Cochin. In: Proceedings of the 6th Kerala Science Congress, Thiruvanthapuram, Kerala, R. Ravikumar (Eds), State Commit Sci Technol Environ 74-77. |
| [49] | Chandran R (1987) Hydrobiological Studies in the Gradient Zone of the Vellar estuary. IV. Benthic Fauna. Mahasagar Bull Nat Ins Oceanog 20: 1-13. |
| [50] | Prabha Devi L (1994) Ecology of Coleroon estuary, Studies on Benthic Fauna. J Mar Biol Ass India 36: 260-266. |
| [51] | Pearson TH, Rosenberg R (1978) Macrobenthos secession in relation to organic enrichment and pollution of the marine environment. Oceanog Mar Biol Annul Rev 16: 229-234. |
| [52] | Kumar RS (2001) Macrobenthos in the Mangrove Ecosystem of Cochin backwaters, Kerala, southwest coast of India. Indian J Mar Sci 24: 56-61. |
| [53] | Jacobsen, D. 2008. Low oxygen pressure as a driving factor for the alttitudinal decline in taxon richness of stream macroinvertebrates. Oecologia (Berl.), 154 (4): 795–807. doi:10.1007/s00442- 007-0877-x. |
| [54] | Koperski, P. 2010. Diversity of macrobenthos in lowland streams: ecological determinants and taxonomic specificity. J. Limnol.69: 1–14. doi:10.3274/JL10-69-1-08. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML