-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2014; 4(1): 45-53
doi:10.5923/j.re.20140401.05
Changes in the Intrinsic Qualities of a Naturally Attenuated Waste Engine Oil Polluted Soil after Exposure to Different Periods of Heat Shock
Beckley Ikhajiagbe 1, Geoffrey O. Anoliefo 1, Osazuwa Omoregbee 2, Paula Osigbemhe 2
1Department of Plant Biology and Biotechnology, University of Benin, Nigeria
2Department of Science Laboratory Technology, University of Benin, Nigeria
Correspondence to: Beckley Ikhajiagbe , Department of Plant Biology and Biotechnology, University of Benin, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study was conducted to investigate the effect of heat shock in the remediation of poly aromatic hydrocarbons (PAHs) and heavy metals of a waste engine oil-polluted soil. This was also subjected to phytoassessment using Vigna unguiculata cv Kano white. Top soil (10kg) was collected and thoroughly mixed with waste engine oil (WEO) on weight basis, to obtain 5% w/w oil-in-soil. The buckets were divided into six sets, each receiving heat treatment at different times; twice per week (2PW), once a week (1PW), once in a fortnight (1PF) and once in a month (1PM). There were 2 controls which did not receive any heat treatment; the unpolluted soil (CTRL) and the polluted soil (NON). Heat was applied by carefully burying a stainless steel container, which received boiling water (100℃) at the designated intervals. The set up was allowed to remain in a well ventilated screen house with inherent room temperature for 3 months. Results showed significant reductions in heavy metal contents after the heat treatment. Heavy metal remediations were better in 1PM and 1PF. Total PAH was the lowest in 1PM (154.51mg/kg), compared to 504.48mg/kg in NON. Comparatively, the most frequent the heat shock received by soil, the lower the rate of remediation. Results of phytoassessment using cowpea also showed improved growth and yield parameters in the less frequently heated oil-polluted buckets, compared to the unheated ones (NON).
Keywords: Bioremediation, Cowpea, Hazard quotient, Heat shock, Natural attenuation, PAH, Petroleum hydrocarbon, Phytoassessment
Cite this paper: Beckley Ikhajiagbe , Geoffrey O. Anoliefo , Osazuwa Omoregbee , Paula Osigbemhe , Changes in the Intrinsic Qualities of a Naturally Attenuated Waste Engine Oil Polluted Soil after Exposure to Different Periods of Heat Shock, Resources and Environment, Vol. 4 No. 1, 2014, pp. 45-53. doi: 10.5923/j.re.20140401.05.
Article Outline
1. Introduction
- Oil exploration and exploitation in many countries have brought about environmental pollution and serious public health concern, which has established the need to create a wide variety of innovative chemical, physical and biological processes that can get rid of hazardous organics from the environment without causing further ecological damage. Remediating a site polluted with hazardous waste materials is a very tedious and complex procedure; and this usually involves a systematic, step-by-step problem solving approach. Bioremediation of contaminated soils is currently regarded as one of the most successful ways to clean up contaminated sites, particularly because it is adjudged ecofriendly. According to bioremediation techniques[1], biological materials and processes are used to reed the soil of contaminants or to reduce the concentration of hazardous pollutants at contaminated sites. Economically, some bioremediation procedures are quite expensive, considering the cost of acquisition of necessary materials as well as remediation design. There exists, however, a very cost effective and very reliable means of bioremediation that utilizes the soil’s inherent intrinsic capabilities for remediating contaminants without having to be necessarily modified by human activity. The process is called natural attenuation.Soil microorganisms play a very important role in the intrinsic bioremediation of contaminants. Themicroorganisms break down contaminants by using them as a food source or cometabolizing them with a food source. Microorganisms can be isolated from almost any environmental conditions. Microbes will adapt and grow at subzero temperatures, as well as extreme heat, desert conditions, in water, with an excess of oxygen, and in anaerobic conditions, with the presence of hazardous compounds or on any waste stream. Field temperatures play a significant role in controlling the nature and extent of hydrocarbon metabolism. Temperature affects the rate of biodegradation, as well as the physical nature and chemical composition of hydrocarbons[1]. The main requirements are an energy source and a carbon source. Because of the adaptability of microbes and other biological systems, these can be used to degrade or remediate environmental hazards.One of the important factors controlling activity and survival of microorganisms is soil temperature. Temperature affects the rate of organic matter decomposition among all the ecological factors. Temperature affects biochemical reactions rates, and the rates of many of them double for each 10℃ rise in temperature. It also determines the rate of biological degradation processes in the soil, as well as the soil moisture content[2]. Temperature increases have been found to increase the rate of degradation of organic compounds in soil[3].Thibault and Elliott[4] reported that the growth of micro-organisms usually doubles for every 10℃ rise in temperature. With increasing temperatures, adsorption decreases; and this have been reported to enhance availability of organic material for microorganisms to use for the degradation[2]. Above a certain temperature, however, the cells die. Although most microorganisms easily adapt to certain temperatures, and hence their bioremediative capabilities in these temperature-dependent situations are therefore unaffected, the present study hopes to investigate the effects of sudden temperature changes in the remediation of these oil-polluted soils. Temperature significantly affects biodegradation of hydrocarbons. Within specific range of temperature, optimum growth and activity of some microorganisms become restricted. Temperature in the thermophilic range (50 to 60℃) was shown to greatly increase decomposition of organic matter, in general. Donald[5] earlier reported that the activities of fungal and bacterial populations are inhibited by high temperatures.Given the fact that natural attenuation of contaminated soils requires a myriad of biological processes in synergism, for example, plant-direct remediation, microbial remediation, biovolatilization, bioleaching, direct or indirect impact by soil micro and macro fauna, etc, it therefore implies that whatever environmental factor, like temperature, that affects any of these processes would definitely affect the efficiency of remediation. The objectives of the present study, therefore, investigate the effects of the frequency of exposure of oil-polluted soil to temperature changes on its intrinsic remediative capabilities.
2. Materials and Methods
2.1. Sample Preparation
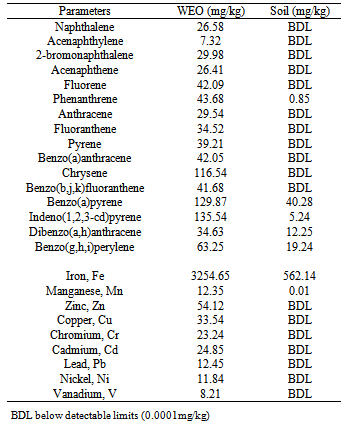
- Top soil (0-10cm), of known chemical properties (Table 1), was collected randomly from a marked area measuring 10 x 10m on a fallow land situated near the Department of Plant Biology and Biotechnology Botanic Garden, University of Benin, Benin City, Nigeria. Thereafter, 10kg sun-dried soil was each measured into large perforated buckets with 8 random perforations made with 2 mm diameter nails at the bottom of each bowl. The buckets were 53.4 cm in height and 21.3 cm in diameter. Soil was thoroughly mixed with waste engine oil (WEO) on weight basis, to obtain 5% w/w oil-in-soil. The buckets were divided into six sets, each receiving heat treatment at different times; twice per week (2PW), once a week (1PW), once in a fortnight (1PF) and once in a month (1PM). There were 2 controls which did not receive any heat treatment; the unpolluted soil (CTRL) and the polluted soil (NON).
2.2. Methodology Adopted
- Heat was applied by carefully burying a stainless steel container of boiling water (100℃), designed to fit the bucket, completely below soil surface (Fig. 1). The container, which was constructed for the purpose of the research, was 1.682 mm thick, 10 cm in diameter and 28 cm in height. The container covered half of the surface area of the soil in order to provide heat to nearly all parts of the surrounding soil. Care was taken to ensure that the water level in the container was same as that of the soil (Fig. 1). Boiling water (100℃) was poured into the container, covered with wooden lid, and then allowed to remain until after 3 hours when the water was cool. Removal was by a syringe. The container remained in position throughout the duration of the research. The set up was allowed to remain in a well ventilated screen house with inherent room temperature for 3 months. Having previously determined the soil’s water holding capacity to be 203 ml/kg soil, moisture requirements of the soils were met by wetting the soil twice a week, equally distributed, with 750 ml of water[6]. At the end of the experiment, the soil was analyzed for heavy metal and polyaromatic hydrocarbon contents.
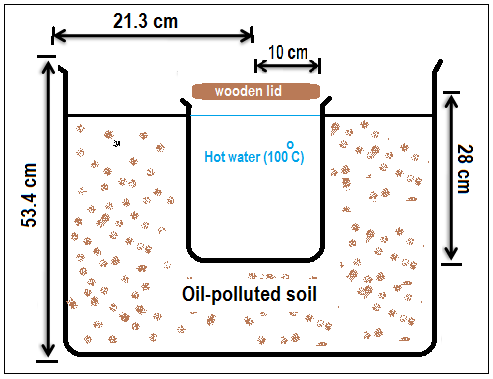 | Figure 1. A cross section of modified bucket stainless steel contained buried in oil-polluted soil |
2.3. Extraction of Micronutrients in Soils by Hydrochloric Acid Method
- Ten (10) g of soil was weighed into a 250 ml plastic bottle. 100 ml of 0.1 m HCI was added, stopper, and then shaken for 30 minutes. The mixture was filtered through Whatman filter paper No.42, and then Fe, Cu, Mn, Zn, Cd, Cr, Pb, Ni, and V were determined in the filtrate by Atomic Absorption Spectrometry.
2.4. Determination of Polyaromatic Hydrocarbon Contents of Polluted Soil by Gas Chromatography (GC)
- A 10 g sample was extracted with methylene chloride (DCM). The extract was filtered through anhydrous sodium sulphate to remove any trapped water molecule. This was followed by a clean- up/fractionation of the sample extract into Aliphatic and Aromatic (PAH) components. Finally, the components were concentrated using a rotary evaporator for GC analysis, using FID as detector. Model of GC used was AGILENT 6890.The GC analysis began by first injecting 1 μL of the sample extract into the GC, and the results calculated as follows:
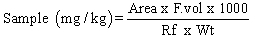 Where, Rf = Response factor = Total Area / Total Concentration, obtained from instrument calibration with standards.Area is obtained from the chromatogram output.F.vol is the final volume of the concentrated extract (in ml)Wt is the initial weight of the homogenized sample (in grams)
Where, Rf = Response factor = Total Area / Total Concentration, obtained from instrument calibration with standards.Area is obtained from the chromatogram output.F.vol is the final volume of the concentrated extract (in ml)Wt is the initial weight of the homogenized sample (in grams)2.5. Identification of Soil Microorganisms
- The soil samples were air-dried and sieved through a 2 mm mesh to remove undesirable material. The dilution series for the soil sample was done by transferring 1 gram of the soil to nine (9 ml) millimetres of sterile distilled water in sterile glass containers as blank. The glass containers were shaken for 5 minutes and was taken as l0-1 dilution factor, 10 ml were then transferred from the 10-1 dilution into another 9 ml blank to obtain a 10-2 dilution and same process of transfer was repeated twice to obtain a dilution factor of 10-4.
2.5.1. Heterotrophic Bacterial and Fungal Counts
- The spread plate method was employed in taking the heterotrophic bacteria counts. One (1) ml of the serially diluted portion of 10-4 of each soil sample was inoculated onto nutrient agar plates for bacteria and Potato dextrose agar plates for fungal counts. The plates were inoculated at room temperature for 24 hours and 72 hours respectively, for bacteria and fungi growth. After incubation colonies were then counted and the colony forming unit (cfu/g) of the soil samples determined.
2.5.2. Isolation of Bacterial and Fungal Oil Degraders
- Bushnell- Haas (BH) medium (MgSO4, 0.20 g/I; CaCl2, 0.02 g/l; K2H,P04, 1 g/l; NH4NO3, 1 g/l; FeCl3, 0.05 g/l; KH2PO4, 1 g/l; pH 7.0, was used as the enrichment medium with 8 % (v/v) filter sterilized oil as the sole carbon source. The medium was dispensed into in 100 ml Erlenmeyer flasks and autoc1aved at 121℃ for 15 minutes. Thereafter, 5 g of each soil sample was inoculated into each flask of the medium and incubated at 130 rpm at room temperature in a HY-4 multifunctional shaker (B. Bran Scientific and Instrument Company, England). After 10 days, 1 ml of enriched media was transferred into freshly prepared enrichment media and incubated under the same conditions as described above. Serial dilutions from the third enrichment process were inoculated onto nutrient agar plates and potato dextrose agar plates for oil-degrading bacterial and funga1 counts respectively by methods described by Cowan and Steel[7] and Cheesebrough[8].
2.6. Computation of Selected Ecotoxicological Statistical Parameters
- Selected parameters used in the study were Hazard quotients (HQ) as well as toxicity equivalency concentrations[6]; the object being to establish at some point whether concentrations of the contaminants after the remediative experiments could still pose any ecological threat. These were calculated as follows;
2.6.1. Hazard Quotient (HQ)
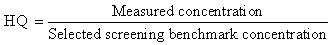 When HQ > 1: Harmful effects are likely due to contaminant in questionWhen HQ = 1: Contaminant alone is not likely to cause ecological riskWhen HQ < 1: Harmful effects are not likelyScreening benchmarks are available at Efroymson et al.[9].
When HQ > 1: Harmful effects are likely due to contaminant in questionWhen HQ = 1: Contaminant alone is not likely to cause ecological riskWhen HQ < 1: Harmful effects are not likelyScreening benchmarks are available at Efroymson et al.[9].2.5.2. Toxic Equivalency (TEQ) for Polycyclic Aromatic Hydrocarbons (PAH).
- TEQ = ƩTi x TEF Where TEQ =Toxic Equivalency
 Ti = PAH concentration in soil
Ti = PAH concentration in soil TEF = Toxic Equivalency factor[10]
TEF = Toxic Equivalency factor[10]2.6. Phytoassessment
- The success of remediation at 3 months was assessed by sowing cowpea (Vigna unguiculata cv. Kano white) in remediated soils. The plants were observed for the following parameters.
2.6.1. Assessment of Seedling Development
- Percentage emergence was calculated as the percentage of seeds that sprouted above soil level of the 5 seeds originally sown per bucket. Heights were also taken by aid of a transparent calibrated ruler at 9 days after sowing (DAS). Dry weights of seedlings were taken at 9 DAS after drying seedlings in an oven at 30℃ for 3 days. Percentage survival was also calculated as the percentage of successfully emerged seedlings compared to total number of seeds sown per bowl.
2.6.2. Other Measurable Growth and Yield Parameters
- Among other measurable growth parameters that were assessed during the study were shoot height, number of primary branches per plant, stem width, leaflet area, root length, number of root nodules per plant (> 3mm in diameters), number of flowers per plant, number of pods harvested per plant, number of seeds per pod, as well as100 seed weight. Grain yield per plant (g/plant) was calculated thus,
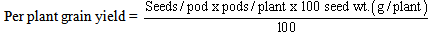 Analysis of variance in completely randomized design was done using the SPSS-15 statistical software, and means were separated by using the Least Significant Difference.
Analysis of variance in completely randomized design was done using the SPSS-15 statistical software, and means were separated by using the Least Significant Difference.3. Results and Discussion
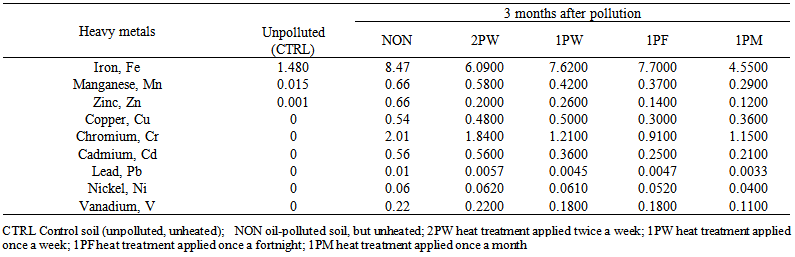
- This study was undertaken with notion to set process conditions to reduce time required for treatment, hence reduce maintenance cost and promote smooth running of the remediation process year round especially under temperature conditions. The chemical composition of waste engine oil and top soil used for the experiment are shown in Table 1. Table 2 shows soil physiochemical parameters at three months after pollution. The total organic carbon was 2.47% in 2PW at pH of 6.85. In NON, organic carbon was 1.63% and total N was 0.13% at pH 6.99. In 1PM, total N was 0.21% at pH 6.59 while in CTRL, it was 0.14% at pH 6.03. In 1PM, Cl was 98.54 mg/kg while it was 83.04mg/kg in CTRL (Table 2).
|
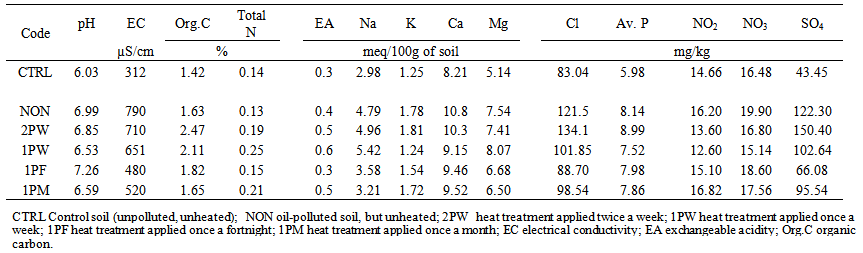 | Table 2. Soil physiochemical parameters at 3 months after soil was polluted with waste engine oil coupled with periodic exposure to heat treatments |
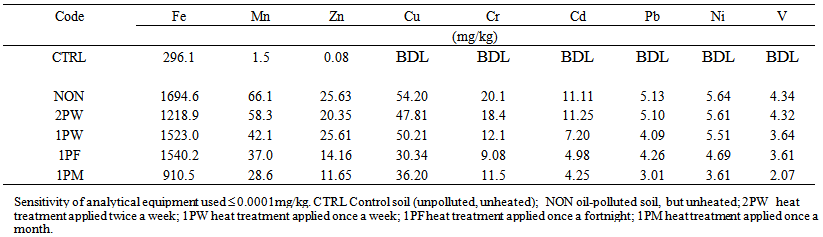 | Table 3. Heavy metal contents of soil at 3 months after pollution with waste engine oil with subsequent periodic exposure to heat treatments |
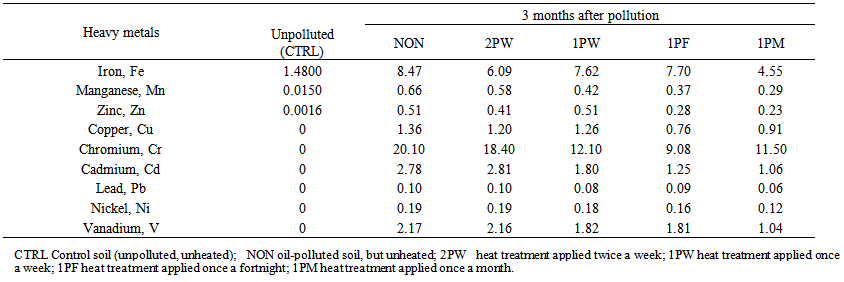 | Table 4. Hazard quotient to determine ecological toxicity of heavy metal components of waste engine oil-polluted soil at 3 months after pollution and regular exposure to heat treatments |
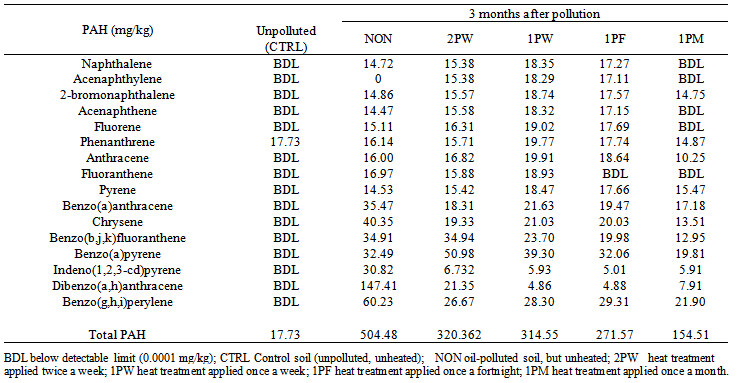 | Table 6. Polyaromatic hydrocarbon contents of oil-polluted soil exposed to periodic heat treatments for 3 months |
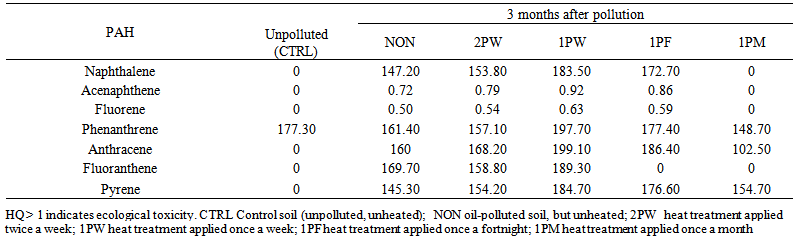 | Table 7. Hazard quotient to determine ecological toxicity of PAH components of waste engine oil-polluted soil at 3 months after pollution and periodic application of heat treatments |
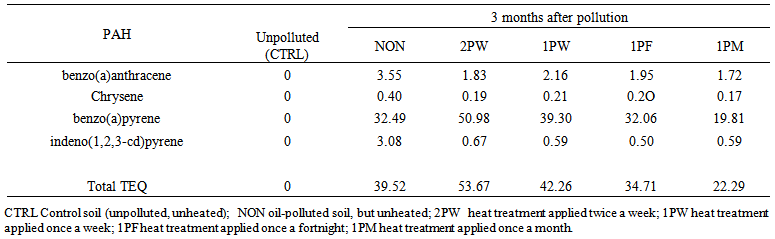 | Table 8. Toxic equivalency (TEQ) concentrations of PAH components of waste engine oil-polluted soil at 3 months after pollution and periodic exposure to heat treatments |
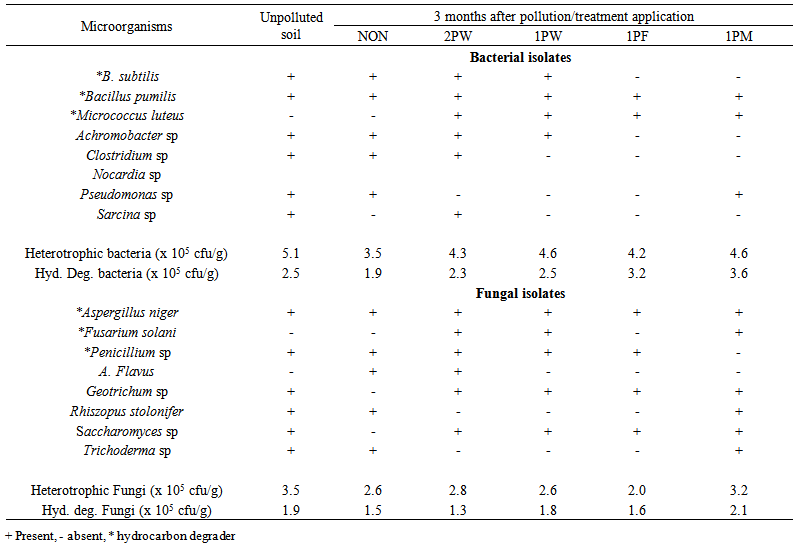 | Table 9. Microbial composition of treated and control soils after waste engine oil pollution and periodic application of heat treatments |
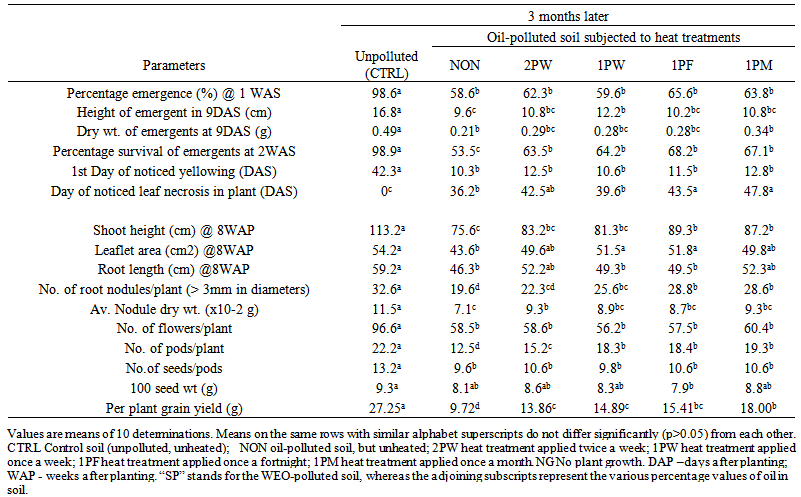 | Table 10. Some growth and yield parameters of Vigna unguiculata cv Kano white sown in heat-treated oil-polluted soils at 3 months after pollution/heat treatments |
4. Conclusions
- It was obvious from the results of the laboratory experiment that temperature is one of the important variables for determining the success of bioremediation of hazardous waste. The results clearly indicated that efficiency of the bioremediation activity was enhanced with high temperature. However, the frequency of exposure of soil to such temperature has been demonstrated in this study as a factor to be reckoned with. Monthly exposure to temperatures as used in the present study has been reported as most effective. The most frequently the heat shock an oil-polluted soil receives, the less effective the rate of remediation. Phytoassessment of the remediated soils also showed comparatively improved growth and yield performances of cowpea in the less frequently heated oil-polluted buckets, compared to the unheated ones. This demonstrates that optimized temperature as well as reduced frequency of exposure speeds up the bioremediation activity in remediation systems.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML