-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2013; 3(6): 169-175
doi:10.5923/j.re.20130306.01
Application of Phyto-Remediation (Sunflower and Vetiver Grass) on Crude Oil Spilled Soil Cultivated to Jute Mallow (Corchorus Olitorius L.)
Udo-Inyang U. Charles 1, Edem I. Dennis 1, N . M. John 2
1Department of Soil Science and Land Resources Management, Faculty of Agriculture, University of Uyo, Uyo, Akwa Ibom State, Nigeria
2Department of Soil Science, Faculty of Agriculture, Forestry & Wildlife Resources Management, University of Calabar, Nigeria
Correspondence to: Edem I. Dennis , Department of Soil Science and Land Resources Management, Faculty of Agriculture, University of Uyo, Uyo, Akwa Ibom State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study assessed the potential phytoremedial use of Tithonia diversifolia (Mexican sunflower) and Vetiveria nigritana (Vetiver Grass) on bioremediation of crude oil affected soils as an option that rendered harmless various contaminants using natural biological activity. It relatively low-cost, low techniques with high public acceptance and can often be carried out on site. The experiment consisted of three treatments; non-remediated soil (A), sunflower (B) and vetiver (C) remediated soils. The treatments in three replicates were laid out in a completely randomized design (CRD) layout. Soil physical conditions were significantly best under vetiver treated soils. Nevertheless, heavy metals concentration after the experiment was in the order of treatments A>B>C. Application of sunflower significantly (p<0.05) reduced the total hydrocarbon content in the soils by 25.59%, while vetiver grass had removal efficiency greater than 41%. Whereas the results of HUB/THBC ratio is in excess of 1% which tends to indicate recent crude oil input into the environment, the differences among the treatments were not significant. The percentage increment in leaf dry weight from 8 to 16 WAP was significantly lower (11%) in pots grown without treatment than those treated; soils treated with sunflower, the percent increment in biomass dry weight from 8 to 16 WAP was 84% and 139% for those treated with vetiver grass for the same period. However, the degree of toxicity on the tested plant was attributed to the direct contact of the toxic volatiles and water soluble hydrocarbons in spilled soil that might have penetrated the tissues of the tender plant.
Keywords: Oil Spill, Phyto-technology, Vetiver, Cleanup, Sunflower, Hydrocarbon, Plant
Cite this paper: Udo-Inyang U. Charles , Edem I. Dennis , Nkereuwem M. John , Application of Phyto-Remediation (Sunflower and Vetiver Grass) on Crude Oil Spilled Soil Cultivated to Jute Mallow (Corchorus Olitorius L.), Resources and Environment, Vol. 3 No. 6, 2013, pp. 169-175. doi: 10.5923/j.re.20130306.01.
Article Outline
1. Background
- Oil contaminated soils are of environmental concern because they are unsuitable for agricultural and recreational use and are potential sources for surface and ground water contamination[1] Petroleum creates unsatisfactoryconditions for plant growth through a number of processes; (i) oil could displace air from soil pore spaces, (ii) an increase in the demand for oxygen brought about by activity of oil-decomposing microorganisms[2] and (iii) petroleum hydrocarbon creates hydrophobic environment, which limits water absorption to plant roots[3].There are several physical-chemical technologies for the treatment of soils contaminated with organic and hazardous materials such as petroleum hydrocarbons. They include vapour extraction, stabilization, solidification, soil flushing, soil washing, thermal desorption, and incineration[4, 5]. These methods have some drawbacks. Most of these techniques are expensive to implement at full scale and require continuous monitoring and control for optimum performance. Some of the techniques simply move the contamination elsewhere and may create significant risks in the excavation, handling and transport of hazardous materials. Also, it is very difficult and increasingly expensive to find new landfill sites for the final disposal of the material. Additionally, they do not usually result in a complete destruction of the contaminants.A better approach than these methods or techniques is to completely destroy the pollutants if possible, or at least to transform them to innocuous substances. Application of Phyto-techniques is alternative methods for the treatment of contaminated soil. These techniques are economically and politically attractive and have shown promising results in the treatment of soil contaminated with organic compounds, particularly with petroleum hydrocarbons[6].Phyto-remediation is an option that offers the possibility to destroy or render harmless various contaminants using natural biological activity. As such, it uses relatively low-cost, low techniques, which generally have a high public acceptance and can often be carried out on site. Phyto-remediation of Mexican sunflower processes have shown to be effective methods that stimulate the biodegradation in contaminated soils[7] Harder[8], estimated that phyto-remediation accounts for 5 to 10 percent of all pollution treatment and has been used successfully in cleaning up the illegal dumping of used engine oil. Lee et al.,[9] reported that oil cleanup methods, especially bioremediation strategies, must take into consideration the local fauna, flora and environmental conditions if they are to make a significant impact.The focus of this study was to assess the effectiveness of sunflower and vetiver remediation technology in cleaning up crude oil contaminated soil for optimum plant production.
2. Materials and Methods
2.1. Site Description
- The study was conducted near the Department of Forestry Arboretum, University of Uyo, Akwa Ibom State, Nigeria. Uyo and its environs, is located between latitudes 40 301 and 50 31N and longitudes 70 311 and 80 201 E[10]. The state has an estimated area of 8, 412 km3. It is characterized by two seasons, a wet season that last for nine (9) months (April –October) and dry season (November-March). The annual rainfall ranges from 2000-3000 mm, while annual temperature varies between 26℃ and 28℃. Relative humidity is high varying from 75-95% with the highest and lowest values in July and January respectively.
2.2. Experiment Layout and Greenhouse Study
 | Figure 1. Showing plants grown on the crude oil spilled contaminated soils (A), cured soil with sunflower (B) and cured soils with vetiver (C) soils |
2.3. Measurement of Growth Characteristics
- When the plants were sufficiently established, six plants were randomly selected from each treatment and tagged for the measurement of growth characteristics at 4, 8 and 16 weeks after planting (WAP) as follows:
2.3.1. Plant Height (cm)
- This was taken as the height of the Corchorus olitorius plant measured to the nearest centimeter from the base to top. The mean height from the randomly selected plants was taken as the score for each treatment.
2.3.2. Number of Leaves Per Plant
- The number of leaves per plant was determined by counting the leaves from plants used to compute the score for each treatment.
2.3.3. Biomass (dry matter) Yield Per Treatment (g)
- Biomass was determined by harvesting the leaf and stem materials after full establishment and oven-dried at 70℃ for 3 days.
2.4. Soil Sample Preparation and Analyses
- All samples were air dried and passed through a 2 mm sieve for routine analyses and 0.5 mm sieve for Organic C and Total N determinations.
2.4.1. Determination of Physico- chemical Properties
- Particle-size distribution was determined by hydrometer method[11]. Soil pH was measured in distilled water (1:2.5 soil:water) with the use of glass electrode pH meter. Available phosphorus was determined using bicarbonate extraction, with acid reductant. The exchangeable cations (Calcium, Ca; Magnesium, Mg; Potassium, K; and Sodium, Na) in the soil were determined by first extracting the soil sediment with 1M NH4 OAc (ammonium acetate) solution[12]. Soil Organic Carbon (SOC) was determined by loss-on-ignition and the standard Van Bemmelen factor (1.724) was used for conversion of SOC into organic matter content[13]. Nitrogen was determined in form of ammonium (NH4-N) and nitrate (NO3-N) by absorbance measurement as described by Nelson[14]. Base saturation (BS) was expressed as the fraction of the negative binding sites occupied by exchangeable cations. It was calculated by summing together the levels of Ca, Mg, K, and Na found in the soil, then expressing this sum as a percentage of the ECEC value as follows:

2.5. Microbial Enumeration of Bacteria and Fungi
- Numbers of viable bacteria and fungi were estimated by the plate count technique. Ten grammes (10g) of 2-mm sieved soil was added to 90ml of sterile distilled water and shaken for 30 minutes. Serial dilutions were prepared up to 106. Total viable counts of culturable aerobic heterotrophic bacteria were obtained by surface plating 1ml of the serial dilution on to sterile nutrient agar while fungi counts were obtained by surface plating 1ml of the serial dilution on to potato dextrose agar (PDA). Culture plates were incubated at room temperature (28 ± 2℃) for 48 hours. Plates yielding counts of 30 - 300 colonies were chosen and the counts obtained were multiplied by the dilution factor and expressed as CFU/g of soil. The morphological characterization (colour and size) of each bacteria isolates and colony characteristics (shape, elevation, edge, surface and pigmentation) were done. Gram staining was also done to determine the reactions of the bacteria to Gram reaction. Fungi identification test was also carried out.
2.6. Statistical Analyses
- All data collected were statistically analysed using analysis of variance (ANOVA) procedure to assess the effect of phyto technology on contaminated soil remediation and plant growth using GLM procedure (SPSS soft ware, Version 17). The means were compared using Fisher’s least significant difference (LSD) test. All tests of significance were made with probability value of 0.05.
3. Results and Discussion
3.1. Effects of Phyto-remediation on Soil Properties
3.1.1. Soil Physical Conditions
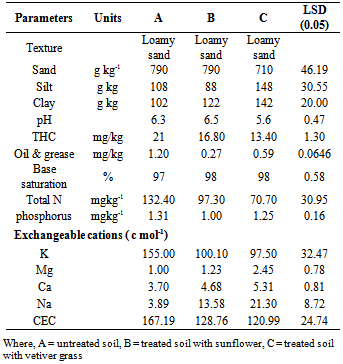
- Soil texture which is considered to have profound effect on many other properties was considered among the physical properties. As shown in Table 1, application of phyto-technology had no effect on the textural class (loamy sand) of the soil, rather the distribution of clay was significantly improved with in the treatment containing vetiver grass (treatment C).
3.1.2. Soil pH (soil reaction)
- Soil pH is one of the most common and important measurements in standard soil analyses. Many soil chemical and biological reactions are controlled by the pH of soil solution in equilibrium with the soil particle surfaces. Soil acidity is a major problem in the production of arable crops in the humid tropical soil of which this location is one. Yields of many crops are highly reduced when the pH values are low (strongly acidic < 5.0)[15]. The results of soil reaction (Table 1) revealed only slight differences in pH among the treatments. The acidity level of the contaminated soil changed from slightly acidic (6.3) to moderately acidic (5.6) when the soil was amended with treatment C, but remained slightly acidic with treatment B (6.5). Obviously, phyto technology reduced the acidic level by 11%, and the soils under this condition can support the optimum performance of most of the arable crops.
|
3.1.3. Total Extractable Hydrocarbon Content (THC) and Oil and Grease in Experimental Soil
- Organic compounds are complex and valuable mixture of compounds of which hydrocarbon is one. An occurrence of THC resulting from crude spilled is alien to soil and their presence affects exchange of oxygen and stains surface soil[16]. The mean concentration of THC was of 21 mgkg-1 in contaminated soil (treatment A), 19.80 and 18.40 mgkg-1 (treatment B and C respectively). Surprisingly, the concentration of THC was significantly higher in the treatment soils. These concentrations could be accounted for manly from biogenic sources of wax and suberins from decay plants that became prominent when submerge in water during watering[17]. Therefore soils receiving treatments B and C are not contaminated by petroleum hydrocarbon. Furthermore, amendment of crude affected soil with sunflower and vetiver reduced the stain THC values by 6 and 14% (removal efficiency of B and C treatments respectively). This shows that the soil was heavily polluted, as evident in the poor growth of Corchorus olitorius especially on the untreated soils. The values of oil and grease showed concentrations that varied from 0.07 to 1.28 mg kg-1 with means values of 1.20, 0.27 and 0.59 mg kg-1 for the respective treatments. Sunflower demonstrated superior reduction efficiency of 344% compared to control and vetiver treated soils.
3.1.4. Total Nitrogen (NH4+, and NH3)
- Total Nitrogen content of a soil gives an indication of the organic nitrogen present in the soil. Most of the Nitrogen in soils is in the organic form as only relatively small quantities occur in NH4+, and NH3; the more available forms. The amount of available nitrogen in the soil is an indication of how suitable conditions in the soils are. A value below 0.1% total Nitrogen is considered low for soils (Isirimah et al., 2003). Between the control and remediated soils, the concentration of Total N in the soil was very high (A=132.40 , B=93.3 and C=70.70 mgkg-1 respectively) The Total Nitrogen values of 0.15, 0.15 - 0.20, and greater than 2.0 mgkg-1 are classified as low, medium, and high respectively [18]. Therefore, the results indicated very high concentration of total N for these soils.
3.1.5. Exchangeable Cations
- The basic cations values in this experiment is not in consonance with the decreasing cation magnitude observed by of Oputa and Udo (1980), that is Ca2++ > Mg2++ > K+ > Na+. The Potassium (K) and cation exchange capacity (CEC) level in the soil followed the decreasing order of Treatment A > B >C. The reverse was true for magnesium, calcium and sodium. In comparing the treatments, it was assumed that Mg, Ca and Na will increase significantly with incremental rate of the phyto application. At 0 t/ha (treatment A), Mg, Ca and Na contents were, 1.00, 3.70, and 3.89 cmolkg-1, respectively. Treatment B (sunflower), increased these cations to 1.23, 4.68 and 13.58 cmolkg-1 respectively. In treatment C (vetiver), the cations further increased respectively to 2.45, 5.31 and 21.30 cmolkg-1. Although, highest CEC was observed in the control soil, the actual exchanges of cations in the soils’ sorption site were hindered due to the presence of stain substances resulting from crude oil spillage. Hendrickx et al.[19], noted that it is crucial to understand the concentration of exchangeable cations as they occur in the area of environmental management as this affects the quality of soil resources. Most of the cations in soils that are available for plant uptake are induced by microbial activities in the soil. Thus CEC availability was also affected by soil environmental factors that affect microbial activities. Apart from stain substances in the contaminated soil, immobilization of magnesium and sodium was largely responsible for the development of acidity in the soils. This soils’ pH varied from slightly to moderately acidic, an indication of low level of Na and Mg (treatment A) which may favour the solubility of Al and Mn thus reducing plant yield. Although Na deficiency has not been identified as a limitation in most of the arable crops production, but magnesium deficiency is common source. This effect of Na and magnesium noticed in the control soil may be due to the rise in the level of exchangeable Al. This according to Rolling et al.[20] usually occurs at low pH and affects crop growth and yield.
3.2. Heavy Metals (Traced elements)
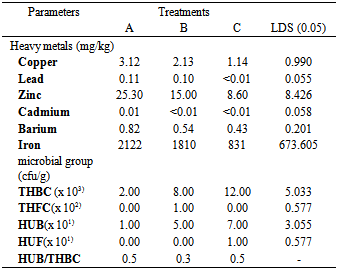
- The mineral elements originate from soil and are dissolved in water for plant roots’ absorption, but those required in small quantity for optimum performance are regarded as traced elements. The concentration of these metals can however be increased to become potential pollutants if heavy metals containing waste products from industrial or domestic activities are introduced into the environment. Concern over the presence of heavy metals in an environment arises from the fact that they cannot be broken down into non toxic forms. Thus once aquatic ecosystems are contaminated by heavy metals; they remain a potential threat for many years[21]. These heavy metals include: Cu, Pb, Zn, Cd, Ba and Fe.
|
3.3. Biological Characteristics
- The microbial community in soil is important because of its relationship to soil fertility and the biochemical cycling of elements. Thus the need to enumerate and isolate major and minor members of the microbial community in soils. Micro organisms are predictable first component of biota in an ecosystem to demonstrate the effect of environmental pollution resulting from any contamination. As shown in Table 2, the soil microbes consisting mainly of fungi and bacteria substantially contribute to the recycling of nutrients within the system. Total heterotrophic bacterial counts (THBC) in untreated treatment (A) averaged 2.0 x 103 cfu/g soil, but on amending the soil with treatment B (sunflower) it averaged 8.0 x 103 cfu/g. In the vetiver treated soils (treatment C), THBC value was 12.0 x 103 cfu/g) was identified. Conversely, there were no heterotrophic fungi counts in the soils except in treatment B, with THFC of 1.0 x 102 cfu/g soil. During the periods under investigation, the Counts for the Hydrocarbon utilizing bacteria (HUB) and fungi (HUF) were equally low among the treatments. Interestingly, the same counts in THFC were found in the HUF, only that 1.0 x 101 cfu/g soil isolated in treatment B was also noticed in treatment C. From the results, HUB/THBC ratio of the treatments is in excess of 1% which is an indication of recent crude oil input into the environment. Also the significant growth differences observed in the treated soils could be attributed to favourable environmental condition consequence upon amendment of soil with plant residues. Soil fauna thrives in relative large number and subsequently brake down complex compounds to simple form that is released into the soil. The bacterial isolates of these soils belongs to the genera Acinetobacter, Alcaligenes, Arthrobacter, Bacillus, and Serratia, while Actinimycetes were of the genera Norcadia. These isolates are typical from soils of the Niger Delta region[22].
3.4. Effects of Crude Oil Polluted Soil on Some Growth Parameters of Jute Mallow Plant
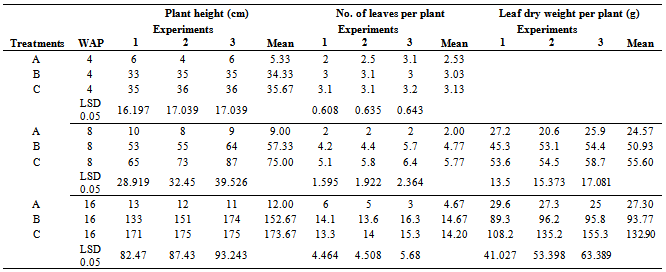
- Inventory of best performed Jute mallow plant in each of the treatments for three experiments are presented in Table 3. Jute mallow plant vegetative growth parameters assessed at 4 WAP showed that averaged plant height and number of leaves per plant varied among experiments and increased significantly among the treatments. At 0 t ha-1 (treatment A), the plant heights were 6,4 and 6 cm (averaged, 5.33 cm), while in treatment B, plants height were 33, 35 and 35cm with mean height of 34.33cm. Also treatment C recorded 35, 36, and 36 cm with average height of 35.67 cm among the respective experiments/treatments. Similarly, number of leaves steadily increased in all the experiments but higher in the treated soils relative to control, indicating that phyto-remediation generally enhanced vigorous plant growth. However, there was no significant difference between treatments B and C. Furthermore, the vegetative growth characteristics measured at 8 and 16 WAP among the three treatments were also significantly better in all pots receiving plant treatment than the control treatment where plant treatment was not applied. The tallest plant height of 152.67 and 173.67 cm were obtained in pots treated with sunflower and vetiver grass respectively. However, there was no significant change in the plant heights among the number of experiments conducted to validate the results in the respective treatments. Although the use of plant substantially promoted plant height, there was no significant increase in the number of leaves at 4 and 8 WAP among the treatments except treatment C at 8 weeks.
|
- At 8 WAP, leaf dry weight significantly varied from 20.6 to 27.2 g at 0 t ha-1 up to 53.1 to 54.4g; and 54.5 to 58.7 g for treatments B and C respectively, with mean values of 24.57g (treatment A), 50.93g (treatment B) and 55.60g (treatment C). At 16 WAP, the mean leaf dry weights were 27.30, 93.77, and 132.9g for the respective treatments A, B, and C, with a range of 25.0 to 29.6g for treatment A, 89.3 to 96.2g for treatment B, and 108.2 to 135.2g for treatment C.The percentage increment in leaf dry weight from 8 to 16 WAP was significantly lower (11%) in pots without treatment than those with treatments; in soils treated with sunflower, the percent increment in leaf dry weight from 8 to 16 WAP was 84% and 139% for those treated with vetiver grass for the same period. However, the general higher percent increments of Jute mallow plant parameters grown on treated soils probably suggest that phyto remediation is effective and it enables plant to attain maximum growth.
4. Findings and Recommendations
- The degree of toxicity on crops was attributed to the direct or contact toxicity of the volatile and water soluble hydrocarbons in spilled soil that may have penetrated the tissues of the tender plant. There were alterations in the physico-chemical properties of the polluted soils treated with sunflower and vetiver grass compared with the control. Application of sunflower significantly (p<0.05) reduced the total hydrocarbon content of the soils by 25.59%, while vetiver grass had removal efficiency greater than 41%. From the above results, it could be concluded that crude oil polluted soils can be reclaimed with the application of phyto technology sourced from sunflower and vetiver grass. Albeit at 2.5 t ha-1, the growth parameters of the cultivated Jute mallow plant gained advantage on soil treated with vetiver grass. With the present poor fertility status of acid sand soil, coupled with scarcity of arable land resource due to oil spillage from exploration of petroleum in the Niger Delta Region of Nigeria, the yield potential of farmers in this study area can be successfully maximized with application of phyto technology of vetiver and sunflower. Therefore, no special management tool is required for this soil when it is contaminated with crude oil. It relatively low-cost, low techniques with high public acceptance and can often be carried out on site.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML