-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Resources and Environment
p-ISSN: 2163-2618 e-ISSN: 2163-2634
2013; 3(5): 155-162
doi:10.5923/j.re.20130305.07
Evaluation of Plants Growing on Lead Mine Spoils: Significance for Abandoned Mine Reclamation in Andhra Pradesh, India
A. Nagaraju1, K. Sunil Kumar1, A. Thejaswi2
1Department of Geology, Sri Venkateswara University, Tirupati, 517 502, India
2Environmental Sciences, School of Distance Education, Kakatiya University, Warangal, 506 009, India
Correspondence to: A. Nagaraju, Department of Geology, Sri Venkateswara University, Tirupati, 517 502, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The strategies for rehabilitation of abandoned mine sites and the need for bioprospecting metal tolerant plants is gaining significance. The aim of this study was to evaluate the potential use of Albiziaamara, Erthroxylum monogynum, Calotropis gigantea, Zizyphus numelaria, Acacia torta, Helecteres isora, Grewia Flavescens, Wrightia tinctoria, and Azadirachta indica for environmental rehabilitation of abandoned mine areas of Andhra Pradesh, India. These plants were sampled from Bandalamottu lead mining area. Leaves, twigs and soil samples were studied for heavy metals viz., Pb, Cu, and Zn and As using acid digestion (1: 1 HNO3 and HCl) followed by measurement of metal concentration using Atomic Absorption Spectrometry. Results of chemical analysis show that these plants can accumulate huge amounts of Pb and As in both leaves and twigs. The leaves of Calotropis giganteaaccumulated the highest concentration of As (5358 μg/l). Wrightia tinctoria accumulated the highest amounts of Pb (1783μg/g) and Cu (608μg/g) in twigs. The results suggest that the plant species analysed could be utilized in the reclamation of Pb polluted mining areas and can be used as model plants for investigating plant tolerance mechanisms.
Keywords: Heavy Metals, Mine Spoils, Accumulation, Bandalamottu Area
Cite this paper: A. Nagaraju, K. Sunil Kumar, A. Thejaswi, Evaluation of Plants Growing on Lead Mine Spoils: Significance for Abandoned Mine Reclamation in Andhra Pradesh, India, Resources and Environment, Vol. 3 No. 5, 2013, pp. 155-162. doi: 10.5923/j.re.20130305.07.
Article Outline
1. Introduction
- Human activities such as mining have continuously increased the concentration of heavy metals in the environment[1, 2, 3, 4]. Waste rocks and tailings are often chemically very unstable and will become sources of pollution. Mining is one of the industrial activities that can locally accounts for greatest and most persistent metal pollution. The ecosystems in the vicinity of mining areas have become subject to considerable disturbance[5]. There are considerable amounts of heavy metals still being transferred off-site even after the closure of a mine[6]. Extraction of metals from sulphide ores commonly results in 9% of the minerals being discarded as tailings[7]. The direct effects of mining wastes include loss of cultivated land, forest or grazing land, and overall loss of production[8]. The indirect effects include air and water pollution. These effects will eventually lead to the loss of biodiversity, amenity and economic wealth since mining activity degrades the environment both directly and indirectly[9]. Plants that accumulate metal and metalloid trace elements to extraordinarily high concentrations in their living biomass have inspired much research worldwide during the last decades[10]. The mine spoil dumps are important sources of metal pollution in the environment as they are characterised by high metal concentrations and particular physical and chemical conditions that make metals environmentally liable and free to move in the surrounding environment, through normal biogeochemical pathways, to sinks such as sediments, soils or biomass[11, 12]. Since 1900 the heavy metal contamination of the biosphere has increased sharply[13]. It poses major environmental and human health problems worldwide[14, 15]. Environmental protection agencies and legislators often insist that mine operators restore mine spoils and tailings because the metal leachates can have a serious impact on the environment. Hence, restoration of mine spoils, tailings and metalliferous soils is a challenging task with potential socio-economic benefit[16]. In Iberian pyrite belt, the plants viz., Cistus salviifolius and Cistus ladanifer have been used for environmental rehabilitation of abandoned mine areas[17]. The data on metallophytes and application to phytoremediation need the attention of all concerned agencies in view of significant progress made in developed nations. This approach is emerging as an innovative tool with greater potential for achieving sustainable development and also to decontaminate metal polluted air, soil, water and for other environmental restoration applications through rhizosphere biotechnology[18, 19]. Metal concentrations in plants not directly related to the total substrate concentration, but mostly on the available metal species in the soil[20, 21] and to their possible involvement in important biological functions[2, 22, 23]. The use of plants to remove pollutants from the environment is known as phytoextraction[24]. A few of the higher plant species have adaptations that enable them to survive and to reproduce in soils heavily contaminated with Zn, Cu, Pb, Cd, Ni and As[25].In the present study, we have focused on certain native plant associations growing on Pb mine spoils of the Bandalamottu area of Agnigundala region of Andhra Pradesh, India and our aims were:(1) To check the role of the uptake by certain native plants to investigate the heavy metal accumulation capacities of plant species viz., Albizia amara, Erthroxylum monogynum, Calotropis gigantea, Zizyphus numelaria, Acacia torta, Helecteres isora, Grewia Flavescens, Wrightia tinctoria, and Azadirachta indica.(2) To assess the metal tolerance strategies adopted by each species with respect to Pb, Cu, and Zn and As and to evaluate the results with special reference to their potential use in developing technologies of phytoremediation of metal contaminated soils.Geology of the study areaLead (Pb) and copper (Cu) ores occur at Bandalmottu, Dhukonda and Nallakonda in the Agnigundala area of Vinukonda mandal in the Guntur district, Andhra Pradesh, India. The area forms the eastern margin of the Cuddapah basin, and the rock types belong to the Nallamalai Series of Upper Cuddapah age. The Bandalamottu area (16° 13' 15'' N: 79° 39' 47'' E) Pb deposit has proved to be the most promising with in the Agnigundala base metal province (Figure 1). In the Bandalamottu block, the rock types are dolomite, cherty dolomite, phyllite with magnetite and chlorite. Earlier workers have studied the geology and genesis of the ore deposit[26, 27].
2. Methodology
2.1. Plants and Soil Sampling
- The sampling of plant and soil materials was carried out by a method described by Brooks[28]. Plants and soil samples were collected during September 2012. Further samples, based on their coverage at the site, together with the associated soil samples were collected from the Bandalamottu lead mine dumps. Plants samples were divided into twigs and leaves, washed gently with deionized distilled water for approximately 3 min to remove soil particles absorbed to the plants. After washing, plant samples were air-dried at room temperature for two weeks and then ground to powders using a Wiley Mill (Wiley Mini-Mill, Model No. 3383-L10, Thomas Scientific, USA). Soil samples were collected from the top horizon (0-20cm) using soil auger. About 36 soil samples weighing about 750 gm for each sample were collected.
2.2. Samples Preparation
- Four samples of leaves and twigs (about 100gm) of each species were used for analysis. The plant samples were ashed at 400°C. Soil samples were sieved at 2mm. About 0.2 g of sample of the finer fraction plant ash and soil was digested in 1:1 HNO3 and HCl and dried in tubes in a water bath. The solutions were made up to 10 ml with distilled water.
2.3. Determinations of Heavy Metals
- Heavy metals in soil and plant samples were determined by atomic absorption spectrometry for Pb, Cu, Zn, and As. For the As determination, 1ml of the solution was mixed with 9ml of 10% HCl containing 10% KI and 5% ascorbic acid. Arsenic concentrations in the samples were determined by hydride-generation atomic absorption spectrometry.
2.4. Data Analysis
- The average concentrations and standard errors were calculated by employing Microcal Origin software for the analytical data of four samples of each species of its organ and soil. These are important because they reflect how much sampling fluctuation a statistic will show, i.e. how good an estimate of the population.
3. Results and Discussion
- The average concentrations of heavy metals in the ash of certain native plant species and soils are shown in Table 1. The data show that there is a variation between species and between organs for element contents. Pb, Zn and As were recorded in lower concentration in plant than soil. There was no consistent correlation for Cu between plant and soil. Further more Pb and Cu were found at higher concentrations in twigs than in leaves. The converse was true for Zn and As
3.1. Lead
- Lead (Pb) is a heavy metal of particular concern with respect to environmental quality and health[29]. As a non-essential trace metal for biological functions, it is highly toxic to plants and animals. Sources of anthropogenic soil contamination by Pb include industrial and agricultural activities such as mining and smelting of metalliferous ores, battery-engine waste, wastewater irrigation, and overuse of chemical fertilizers and pesticides[30]. Its uptake is passive, and its translocation from roots to other plant organs is generally low[31]. High concentration of lead (Pb) 1783 (µg/g), a concentration was observed in twigs of Wrightia tinctoria. The average concentrations of Pb in soils ranged from 11332 to 95533 (µg/g). Xiong[32] has observed in Sonchus oleraceus L that the leaves contained much lower Pb than stems and roots. He states that Pb concentrations in leaves, stems, and roots were positively correlated with one another. Barry and Clark[33] found Pb concentrations even up to 20,000 mg kg-1 in shoots of Minuartia species growing on a soil concentration of 41250 mg kg-1 Pb in the soil in the Pennines mountains in England.The lead (Pb) tolerance potential of six legume species grown on lead ore tailings was studied by Sudhakar et al.,[34]. Root and shoot growth data revealed that bengal gram and cowpea adapted to high lead (Pb) concentrations better than the other legumes. Furthermore, the availability of Pb to plants is low, and relatively large differences in soil contents result in only small increases in plant concentrations[35]. Even Pb known as an element which occurs predominantly at surface of plants - can be accumulated in the stem wood with considerable amounts[36]. However, the accumulation rate is very low and thus contents were only found in the stem of trees from ore wastes.In mining areas heavy metal contents of the assimilation organs are influenced by soil contamination and direct atmospheric input both. Atmospheric input usually causes an accumulation at the surface of the leaves increasing with the time of exposition. This phenomenon is well known for lead, which forms rather insoluble compounds[36]. However, increased Pb-contents in the needles of old trees from ore wastes indicate also a soil uptake and a translocation to the needles. The translocation about these long distances is very ineffective, obviously. Hirschfeldia incana, from metalliferous mine spoils in Morocco, as a Pb accumulator plant. H. incana exhibited high Pb accumulation in mine soils. The major Pb accumulation occurred in the roots and a part of Pb translocated from the roots to the shoots, even to the siliques[37].
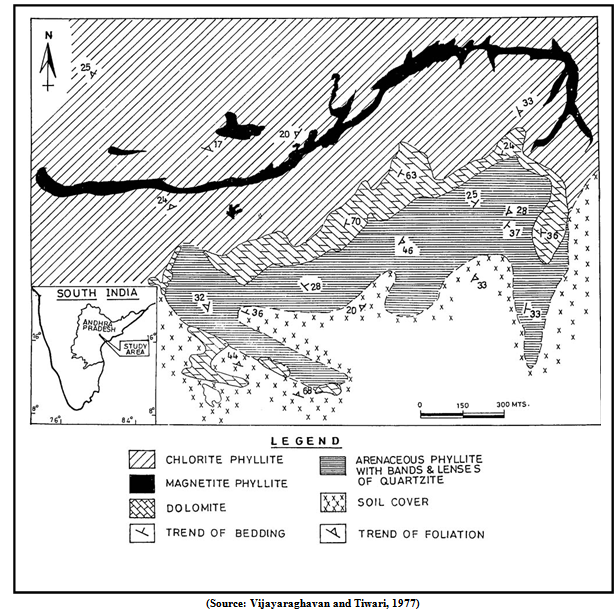 | Figure 1. Geological map of Bandalamottu area |
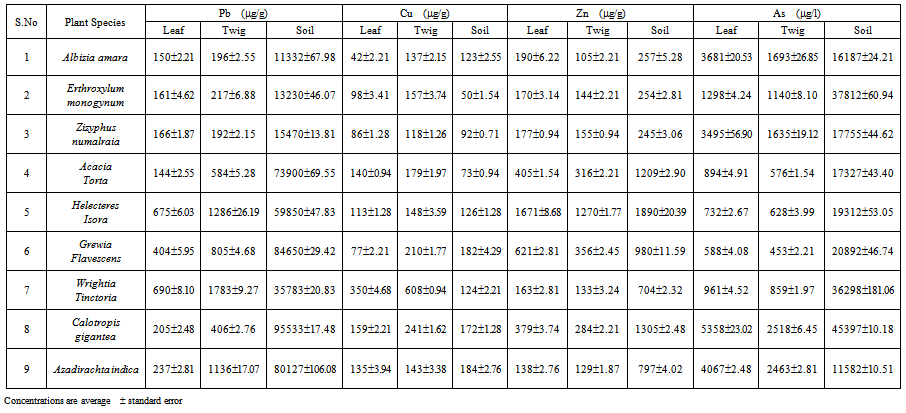 | Table 1. Average concentration of various heavy metals in certain plants and their soils growing on mine spoils |
3.2. Zinc
- Zinc (Zn) is an essential element in all organisms and plays an important role in the biosynthesis of enzymes, auxins, and some proteins[22]. Typical concentrations of Zn in plants are in the range of 10 to 100 ppm[38, 39]. Zn concentrations in the leaves of Thlaspi calaminare and Viola calaminaria can reach 3.5 and 1.0%, respectively. The former species, together with others regarded for many years as part of the Thlaspi alpestre complex, is now properly referred to as T. caerulescens[40]. Several other Thlaspi species from lead/zinc-mineralized soils have been reportedwith up to 2.0% Zn[41]. The distribution of T. caerulescens in Britain and Belgium is strongly linked to lead/zinc mines[42, 43]. In the present study, very high concentrations of Zn with a mean value of about 1671 (µg/g) were accumulated in the leaves of Helecteres isora. Zn concentrations in soils are ranging from 245 to 1890 (µg/g). Robinson et al.,[44] have studied the uptake of Cd, Zn, Pb and Mn by the hyperaccumulator Thlaspi caerulescens was studied by pot trials(utilised metal-contaminated soil) growing over Pb/Zn base-metal mine wastes at Les Malines in the south of France.
3.3. Copper
- Copper (Cu) is required in very small amounts, about 5 to 20 ppm in plant tissue being adequate for normal growth, while less than 4 ppm is considered deficient and above 20 ppm is considered toxic[45]. Most of the hyperaccumulators of copper discovered to date are confined to Shaba Province in Zaïre (now the Democratic Republic of Congo) and the Copper Belt of northwest Zambia[46]. The plants of hyperaccumulators of copper, several of which also hyperaccumulate cobalt, have been reported from Africa, occurring principally in advanced families such as Lamiaceae and Scrophulariaceae[47]. Particularly elevated Cu concentrations (up to 13,700 μg/g) have been found in Aeollanthus biformifolius De Wild. (Lamiaceae), a dwarf peren-nial herb which also hyperaccumulates cobalt, endemic to the southern part of the Shaban Copper Arc[48]. Concentrations of copper (Cu) about 350 and 608 (µg/g) were accumulated in leaves and twigs of Wrightia tinctoria. The twigs generally contained more copper than leaves. Pal and Sindhupe[49] found copper (Cu) concentrations in certain plants viz., Hyptis suaveolens and Sterculia urens occurring in the Malanjkhand area of Madhya Pradesh in India containing more amounts of Cu in twigs than in leaves. In soils the concentrations of Cu ranged from 50 to 184 (µg/g). Pb and Cu concentrations exhibited similar trends among the species and organs. Dahmani-Muller et al.,[50] have also observed such trends in the plant species Armeria maritima spp. Halleri occurring near a metal smelter.
3.4. Arsenic
- Arsenic (As) has been an element that has been the focus of considerable environmental concern in recent years because of its toxicity and carcinogenic properties[51, 52]. The role of Chinese brake fern (Pteris vittata) as an arsenic hyperaccumulator lead to the development ofphytoremediation of arsenic[53]. Arsenic (As) concentrations in plants from areas influenced by mining activities have already been reported[54]. It has also been reported that arsenic(As) bioaccumulates in oak leaves from the Colline Mealifere (Tuscany) mining area of Italy[55]. The growth of Populus and Salix and trace element uptake were investigated in both pot and field trial by Vamerali et al.[56]. The most significant finding was of coarse and fine roots proliferation in surface layers that provided a significant sink for trace elements.In the present study, arsenic (As) concentrations in soils are ranged from 11582 (µg/l) to 45397 (µg/l). Higher concentrations of arsenic (As) were accumulated in leaves than twigs in all the plant species studied. Calotropis gigantea has accumulated arsenic (As) concentration of about 5358 (µg/l) in their leaves. Calotropis gigantea has been found suitable for phytoremediation of radio strontium (90Sr) and radio cesium (137Cs) contaminated sites[57]. Furthermore, Azadirachta indica also accumulated high concentrations of arsenic (As) in both organs. The accumulation of arsenic (As) in plants is derived mainly derived from pyritic minerals. Similar observation has been noticed by Bech et al.[54] in certain plants grown near copper mine in Northern Peru. Plants under natural conditions take up metals only to an extent that does not exceed the pool of the metals in the soil that is readily accessible for plant roots. This plant-available metal pool in the rhizosphere of hyperaccumulator species is likely to be sustained by recycling of metals via sequence of the shoot system. In addition to genetic variation, differential uptake of metals by specimens of the same species may therefore be related to the amount of readily available metals in the soil[58]. The vegetation growing on tailings is subjected to a harsh environment, especially the various adverse edaphic factors in addition to besides the elevated heavy metals. Heavy metals can cause severe phytotoxic action, and may act as a powerful force for the evolution of tolerant populations. It is easy to identify metal tolerant species from the natural vegetation of metalliferous mine wastes[42, 59, 60, 61]. Mine tailings impose various adverse effects on plant growth through high levels of various heavy metals and other elements in toxic concentrations, low amounts major plant nutrients, acidity, salinity and alkalinity[62]. This suggests that populations of plant species growing on Pb mine tailings can tolerate elevated metal concentrations. Therefore, selection of such appropriate plant species which can establish, grow and colonise metal-contaminated soils is important for successful reclamation of degraded mine sites [8]. Although some tolerant plants are now commercially available for restoration of metalliferous wastelands, there has been continuous interest in searching for native tolerant plants which can adapt to local climatic conditions and are able to colonise metal-enriched soils for use in land reclamation[63, 34, 64, 65].Successful establishment and colonization of several pioneer plant species tolerant to Pb/Zn mine spoils have been demonstrated. The tolerant species have included grasses viz., Vetiveria zizanioides[66], the shrubby legume Sesbania rostrata[67], and the woody legume Leucaena leucocephala [68].
4. Conclusions
- This study was conducted to screen certain native plants growing on a contaminated site of Bandalamottu to determine their potential for metal accumulation. Among those plant species studied from the contaminated site, Wrightia tinctoria, Calotropis gigantea, Helecteres isora and Azadirachta indica are considered as the most promising species for phytoextraction of heavy metal-contaminated sites. The most successful populations at each site were those with highest tolerance to the metals occurring on the waste, provided the species was appropriately adapted to the other soil conditions. So, these plant species must have developed tolerance mechanisms which enable them to survive on the toxic soils.From the above results, it is clear that rocks viz. dolomite, phyllite, magnetite and minerals like amphiboles, chlorite, calcite, feldspar, mica, pyrite, and quartz are most likely responsible for the release of elements. The study has pointed out the plant species viz., Wrightia tinctoria, Calotropis gigantea, Helecteres isora and Azadirachta indica as particularly able to tolerate the large amount of these elements. Further, the nutrients present in mill tailings can be used for the ecological restoration of dumps. The plants must be tolerant to toxic metals and should be ideal pioneer species to accelerate ecological succession of man-made habitats In all cases tolerance has been shown to be element specific although plants from mine wastes which contain multiple toxicities have evolved tolerance to all of the metals involved. Because of the diversity of heavy metals present in many ore bodies and the diverse range of heavy metal dispersion mechanisms, it therefore follow that environmental managers at mine sites, who are responsible for impounded mining wastes, must be as well versed in the biogeochemical cycling and accumulation of these metals.
ACKNOWLEDGEMENTS
- Author is grateful to University Grants Commission (UGC), New Delhi for funding the form of major research project (Project No. 39-140) to carry out the present research program.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML